IL-10 Mediated Immunomodulation Limits Subepithelial Fibrosis and Repairs Airway Epithelium in Rejecting Airway Allografts
- PMID: 34069395
- PMCID: PMC8158696
- DOI: 10.3390/cells10051248
IL-10 Mediated Immunomodulation Limits Subepithelial Fibrosis and Repairs Airway Epithelium in Rejecting Airway Allografts
Abstract
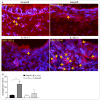
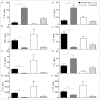
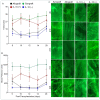
Interleukin-10 plays a vital role in maintaining peripheral immunotolerance and favors a regulatory immune milieu through the suppression of T effector cells. Inflammation-induced microvascular loss has been associated with airway epithelial injury, which is a key pathological source of graft malfunctioning and subepithelial fibrosis in rejecting allografts. The regulatory immune phase maneuvers alloimmune inflammation through various regulatory modulators, and thereby promotes graft microvascular repair and suppresses the progression of fibrosis after transplantation. The present study was designed to investigate the therapeutic impact of IL-10 on immunotolerance, in particular, the reparative microenvironment, which negates airway epithelial injury, and fibrosis in a mouse model of airway graft rejection. Here, we depleted and reconstituted IL-10, and serially monitored the phase of immunotolerance, graft microvasculature, inflammatory cytokines, airway epithelium, and subepithelial collagen in rejecting airway transplants. We demonstrated that the IL-10 depletion suppresses FOXP3+ Tregs, tumor necrosis factor-inducible gene 6 protein (TSG-6), graft microvasculature, and establishes a pro-inflammatory phase, which augments airway epithelial injury and subepithelial collagen deposition while the IL-10 reconstitution facilitates FOXP3+ Tregs, TSG-6 deposition, graft microvasculature, and thereby favors airway epithelial repair and subepithelial collagen suppression. These findings establish a potential reparative modulation of IL-10-associated immunotolerance on microvascular, epithelial, and fibrotic remodeling, which could provide a vital therapeutic option to rescue rejecting transplants in clinical settings.
Keywords: TSG-6; immunotolerance; interleukin-10; subepithelial fibrosis.
Conflict of interest statement
All authors declare that they have no other competing interests as defined by
Figures



Similar articles
-
Targeting Interleukin-10 Restores Graft Microvascular Supply and Airway Epithelium in Rejecting Allografts.Int J Mol Sci. 2022 Jan 23;23(3):1269. doi: 10.3390/ijms23031269. Int J Mol Sci. 2022. PMID: 35163192 Free PMC article.
-
CTLA4-Ig mediated immunosuppression favors immunotolerance and restores graft in mouse airway transplants.Pharmacol Res. 2022 Apr;178:106147. doi: 10.1016/j.phrs.2022.106147. Epub 2022 Feb 26. Pharmacol Res. 2022. PMID: 35227891
-
iPSC-derived MSC therapy induces immune tolerance and supports long-term graft survival in mouse orthotopic tracheal transplants.Stem Cell Res Ther. 2019 Sep 23;10(1):290. doi: 10.1186/s13287-019-1397-4. Stem Cell Res Ther. 2019. PMID: 31547869 Free PMC article.
-
FOXP3+ regulatory T cells: from suppression of rejection to induction of renal allograft tolerance.Transpl Immunol. 2012 Jan;26(1):1-10. doi: 10.1016/j.trim.2011.08.009. Epub 2011 Sep 13. Transpl Immunol. 2012. PMID: 21939765 Review.
-
The dual role of tissue regulatory T cells in tissue repair: return to homeostasis or fibrosis.Front Immunol. 2025 Mar 6;16:1560578. doi: 10.3389/fimmu.2025.1560578. eCollection 2025. Front Immunol. 2025. PMID: 40114929 Free PMC article. Review.
Cited by
-
Targeting Interleukin-10 Restores Graft Microvascular Supply and Airway Epithelium in Rejecting Allografts.Int J Mol Sci. 2022 Jan 23;23(3):1269. doi: 10.3390/ijms23031269. Int J Mol Sci. 2022. PMID: 35163192 Free PMC article.
-
Newborns from Mothers Who Intensely Consumed Sucralose during Pregnancy Are Heavier and Exhibit Markers of Metabolic Alteration and Low-Grade Systemic Inflammation: A Cross-Sectional, Prospective Study.Biomedicines. 2023 Feb 21;11(3):650. doi: 10.3390/biomedicines11030650. Biomedicines. 2023. PMID: 36979631 Free PMC article.
-
Glycosaminoglycan, Antimicrobial Defence Molecule and Cytokine Appearance in Tracheal Hyaline Cartilage of Healthy Humans.J Funct Morphol Kinesiol. 2022 Jul 21;7(3):55. doi: 10.3390/jfmk7030055. J Funct Morphol Kinesiol. 2022. PMID: 35893329 Free PMC article.
-
Monitoring regulatory T cells as a prognostic marker in lung transplantation.Front Immunol. 2023 Sep 25;14:1235889. doi: 10.3389/fimmu.2023.1235889. eCollection 2023. Front Immunol. 2023. PMID: 37818354 Free PMC article. Review.
-
Bronchial anastomotic complications as a microvascular disruption in a mouse model of airway transplantation.Front Immunol. 2025 May 14;16:1567657. doi: 10.3389/fimmu.2025.1567657. eCollection 2025. Front Immunol. 2025. PMID: 40438113 Free PMC article. Review.
References
-
- Heim C., Khan M.A., von Silva-Tarouca B., Kuckhahn A., Stamminger T., Ramsperger-Gleixner M., Nicolls M.R., Weyand M., Ensminger S.M. Preservation of Microvascular Integrity in Murine Orthotopic Tracheal Allografts by Clopidogrel. Transplantation. 2019;103:899–908. doi: 10.1097/TP.0000000000002571. - DOI - PubMed
-
- Jiang X., Nguyen T.T., Tian W., Sung Y.K., Yuan K., Qian J., Rajadas J., Sallenave J.M., Nickel N.P., de Jesus Perez V., et al. Cyclosporine Does Not Prevent Microvascular Loss in Transplantation but Can Synergize With a Neutrophil Elastase Inhibitor, Elafin, to Maintain Graft Perfusion During Acute Rejection. Am. J. Transplant. 2015 doi: 10.1111/ajt.13189. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

