Modulation of Cyclic AMP Levels in Fallopian Tube Cells by Natural and Environmental Estrogens
- PMID: 34069403
- PMCID: PMC8158772
- DOI: 10.3390/cells10051250
Modulation of Cyclic AMP Levels in Fallopian Tube Cells by Natural and Environmental Estrogens
Abstract
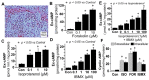
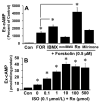
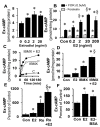
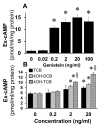
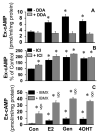
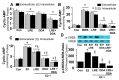
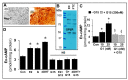
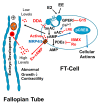
Autocrine/paracrine factors generated in response to 17β-estradiol (E2) within the fallopian tube (FT) facilitate fertilization and early embryo development for implantation. Since cyclic AMP (cAMP) plays a key role in reproduction, regulation of its synthesis by E2 may be of biological/pathophysiological relevance. Herein, we investigated whether cAMP production in FT cells (FTCs) is regulated by E2 and environmental estrogens (EE's; xenoestrogens and phytoestrogens). Under basal conditions, low levels of extracellular cAMP were detectable in bovine FTCs (epithelial cells and fibroblasts; 1:1 ratio). Treatment of FTCs with forskolin (AC; adenylyl cyclase activator), isoproterenol (β-adrenoceptor agonist) and IBMX (phosphodiesterase (PDE) inhibitor) dramatically (>10 fold) increased cAMP; whereas LRE1 (sAC; soluble AC inhibitor) and 2',5'-dideoxyadenosine (DDA; transmembrane AC (tmAC)) inhibitor decreased cAMP. Comparable changes in basal and stimulated intracellular cAMP were also observed. Ro-20-1724 (PDE-IV inhibitor), but not milrinone (PDE-III inhibitor) nor mmIBMX (PDE-I inhibitor), augmented forskolin-stimulated cAMP levels, suggesting that PDE-IV dominates in FTCs. E2 increased cAMP levels and CREB phosphorylation in FTCs, and these effects were mimicked by EE's (genistein, 4-hydroxy-2',4',6'-trichlorobiphenyl, 4-hydroxy-2',4',6'-dichlorobiphenyl). Moreover, the effects of E2 and EE were blocked by the tmAC inhibitor DDA, but not by the ERα/β antagonist ICI182780. Moreover, BAPTA-AM (intracellular-Ca2+ chelator) abrogated the effects of E2, but not genistein, on cAMP suggesting differential involvement of Ca2+. Treatment with non-permeable E2-BSA induced cAMP levels and CREB-phosphorylation; moreover, the stimulatory effects of E2 and EEs on cAMP were blocked by G15, a G protein-coupled estrogen receptor (GPER) antagonist. E2 and IBMX induced cAMP formation was inhibited by LRE1 and DDA suggesting involvement of both tmAC and sAC. Our results provide the first evidence that in FTCs, E2 and EE's stimulate cAMP synthesis via GPER. Exposure of the FT to EE's and PDE inhibitors may result in abnormal non-cyclic induction of cAMP levels which may induce deleterious effects on reproduction.
Keywords: endocrine disruptors; fallopian tube; fertilization; hormones; infertility.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. There is no conflict of interest, which could be perceived as prejudicing the impartiality of the research reported.
Figures



References
-
- Almiñana C., Tsikis G., Labas V., Uzbekov R., da Silveira J.C., Bauersachs S., Mermillod P. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implications for the gametes-oviduct interactions and the environment of the potential embryo. BMC Genom. 2018;19:622. doi: 10.1186/s12864-018-4982-5. - DOI - PMC - PubMed
-
- Sayner S.L., Choi C.-S., Maulucci M.E., Ramila K.C., Zhou C., Scruggs A.K., Yarbrough T., Blair L.A., King J.A., Seifert R., et al. 2019 Extracellular vesicles: Another compartment for the second messenger, cyclic adenosine monophosphate. Am. J. Physiol. Lung Cell Mol. Physiol. 2019:L691–L700. doi: 10.1152/ajplung.00282.2018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

