Gut Microbiota, in the Halfway between Nutrition and Lung Function
- PMID: 34069415
- PMCID: PMC8159117
- DOI: 10.3390/nu13051716
Gut Microbiota, in the Halfway between Nutrition and Lung Function
Abstract
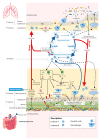
The gut microbiota is often mentioned as a "forgotten organ" or "metabolic organ", given its profound impact on host physiology, metabolism, immune function and nutrition. A healthy diet is undoubtedly a major contributor for promoting a "good" microbial community that turns out to be crucial for a fine-tuned symbiotic relationship with the host. Both microbial-derived components and produced metabolites elicit the activation of downstream cascades capable to modulate both local and systemic immune responses. A balance between host and gut microbiota is crucial to keep a healthy intestinal barrier and an optimal immune homeostasis, thus contributing to prevent disease occurrence. How dietary habits can impact gut microbiota and, ultimately, host immunity in health and disease has been the subject of intense study, especially with regard to metabolic diseases. Only recently, these links have started to be explored in relation to lung diseases. The objective of this review is to address the current knowledge on how diet affects gut microbiota and how it acts on lung function. As the immune system seems to be the key player in the cross-talk between diet, gut microbiota and the lungs, involved immune interactions are discussed. There are key nutrients that, when present in our diet, help in gut homeostasis and lead to a healthier lifestyle, even ameliorating chronic diseases. Thus, with this review we hope to incite the scientific community interest to use diet as a valuable non-pharmacological addition to lung diseases management. First, we talk about the intestinal microbiota and interactions through the intestinal barrier for a better understanding of the following sections, which are the main focus of this article: the way diet impacts the intestinal microbiota and the immune interactions of the gut-lung axis that can explain the impact of diet, a key modifiable factor influencing the gut microbiota in several lung diseases.
Keywords: diet; gut microbiota; gut-lung axis; immune system; lung function; nutrition respiratory health.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

