Polyphenols Modulating Effects of PD-L1/PD-1 Checkpoint and EMT-Mediated PD-L1 Overexpression in Breast Cancer
- PMID: 34069461
- PMCID: PMC8159140
- DOI: 10.3390/nu13051718
Polyphenols Modulating Effects of PD-L1/PD-1 Checkpoint and EMT-Mediated PD-L1 Overexpression in Breast Cancer
Abstract
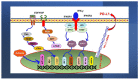
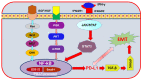
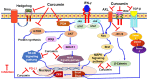
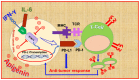
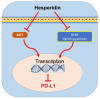
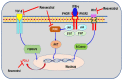
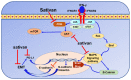
Investigating dietary polyphenolic compounds as antitumor agents are rising due to the growing evidence of the close association between immunity and cancer. Cancer cells elude immune surveillance for enhancing their progression and metastasis utilizing various mechanisms. These mechanisms include the upregulation of programmed death-ligand 1 (PD-L1) expression and Epithelial-to-Mesenchymal Transition (EMT) cell phenotype activation. In addition to its role in stimulating normal embryonic development, EMT has been identified as a critical driver in various aspects of cancer pathology, including carcinogenesis, metastasis, and drug resistance. Furthermore, EMT conversion to another phenotype, Mesenchymal-to-Epithelial Transition (MET), is crucial in developing cancer metastasis. A central mechanism in the upregulation of PD-L1 expression in various cancer types is EMT signaling activation. In breast cancer (BC) cells, the upregulated level of PD-L1 has become a critical target in cancer therapy. Various signal transduction pathways are involved in EMT-mediated PD-L1 checkpoint overexpression. Three main groups are considered potential targets in EMT development; the effectors (E-cadherin and Vimentin), the regulators (Zeb, Twist, and Snail), and the inducers that include members of the transforming growth factor-beta (TGF-β). Meanwhile, the correlation between consuming flavonoid-rich food and the lower risk of cancers has been demonstrated. In BC, polyphenols were found to downregulate PD-L1 expression. This review highlights the effects of polyphenols on the EMT process by inhibiting mesenchymal proteins and upregulating the epithelial phenotype. This multifunctional mechanism could hold promises in the prevention and treating breast cancer.
Keywords: Epithelial-to-Mesenchymal Transition; breast cancer; polyphenols; programmed death-ligand 1; triple-negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
PD-L1 Expression Promotes Epithelial to Mesenchymal Transition in Human Esophageal Cancer.Cell Physiol Biochem. 2017;42(6):2267-2280. doi: 10.1159/000480000. Epub 2017 Aug 17. Cell Physiol Biochem. 2017. PMID: 28848143
-
Chemotherapy enhances programmed cell death 1/ligand 1 expression via TGF-β induced epithelial mesenchymal transition in non-small cell lung cancer.Oncol Rep. 2017 Oct;38(4):2277-2284. doi: 10.3892/or.2017.5894. Epub 2017 Aug 9. Oncol Rep. 2017. PMID: 28849209
-
Immune checkpoint molecules are regulated by transforming growth factor (TGF)-β1-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma.Int J Med Sci. 2021 Apr 22;18(12):2466-2479. doi: 10.7150/ijms.54239. eCollection 2021. Int J Med Sci. 2021. PMID: 34104078 Free PMC article.
-
Determining Factors in the Therapeutic Success of Checkpoint Immunotherapies against PD-L1 in Breast Cancer: A Focus on Epithelial-Mesenchymal Transition Activation.J Immunol Res. 2021 Jan 7;2021:6668573. doi: 10.1155/2021/6668573. eCollection 2021. J Immunol Res. 2021. PMID: 33506060 Free PMC article. Review.
-
Communication between EMT and PD-L1 signaling: New insights into tumor immune evasion.Cancer Lett. 2020 Jan 1;468:72-81. doi: 10.1016/j.canlet.2019.10.013. Epub 2019 Oct 9. Cancer Lett. 2020. PMID: 31605776 Review.
Cited by
-
Formosanin C inhibits triple-negative breast cancer progression by suppressing the phosphorylation of STAT3 and the polarization of M2 macrophages.Breast Cancer Res Treat. 2025 May;211(1):71-89. doi: 10.1007/s10549-025-07623-8. Epub 2025 Feb 14. Breast Cancer Res Treat. 2025. PMID: 39953272
-
Role of FHOD1 in tumor cells and tumor immune microenvironment.Front Immunol. 2025 Apr 29;16:1514488. doi: 10.3389/fimmu.2025.1514488. eCollection 2025. Front Immunol. 2025. PMID: 40364836 Free PMC article. Review.
-
Revolutionizing Skin Cancer Treatment: The Rise of PD-1/PDL-1 and CTLA-4 as Key Therapeutic Targets.Curr Drug Targets. 2024;25(15):1012-1026. doi: 10.2174/0113894501320281240822052657. Curr Drug Targets. 2024. PMID: 39257156 Review.
-
What role does PDL1 play in EMT changes in tumors and fibrosis?Front Immunol. 2023 Aug 15;14:1226038. doi: 10.3389/fimmu.2023.1226038. eCollection 2023. Front Immunol. 2023. PMID: 37649487 Free PMC article. Review.
-
SNAIL1: Linking Tumor Metastasis to Immune Evasion.Front Immunol. 2021 Nov 30;12:724200. doi: 10.3389/fimmu.2021.724200. eCollection 2021. Front Immunol. 2021. PMID: 34917071 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

