Formulative Study and Intracellular Fate Evaluation of Ethosomes and Transethosomes for Vitamin D3 Delivery
- PMID: 34069489
- PMCID: PMC8161393
- DOI: 10.3390/ijms22105341
Formulative Study and Intracellular Fate Evaluation of Ethosomes and Transethosomes for Vitamin D3 Delivery
Abstract
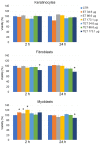
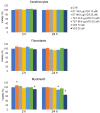
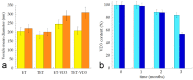
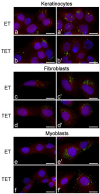
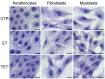
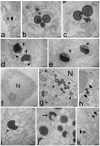
In this pilot study, ethosomes and transethosomes were investigated as potential delivery systems for cholecalciferol (vitamin D3), whose deficiency has been correlated to many disorders such as dermatological diseases, systemic infections, cancer and sarcopenia. A formulative study on the influence of pharmaceutically acceptable ionic and non-ionic surfactants allowed the preparation of different transethosomes. In vitro cytotoxicity was evaluated in different cell types representative of epithelial, connective and muscle tissue. Then, the selected nanocarriers were further investigated at light and transmission electron microscopy to evaluate their uptake and intracellular fate. Both ethosomes and transethosomes proven to have physicochemical properties optimal for transdermal penetration and efficient vitamin D3 loading; moreover, nanocarriers were easily internalized by all cell types, although they followed distinct intracellular fates: ethosomes persisted for long times inside the cytoplasm, without inducing subcellular alteration, while transethosomes underwent rapid degradation giving rise to an intracellular accumulation of lipids. These basic results provide a solid scientific background to in vivo investigations aimed at exploring the efficacy of vitamin D3 transdermal administration in different experimental and pathological conditions.
Keywords: cell culture; cholecalciferol; cryogenic transmission electron microscopy; in vitro test; light microscopy; lipid nanocarriers; transmission electron microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

