The Role of Ceramide Metabolism and Signaling in the Regulation of Mitophagy and Cancer Therapy
- PMID: 34069611
- PMCID: PMC8161379
- DOI: 10.3390/cancers13102475
The Role of Ceramide Metabolism and Signaling in the Regulation of Mitophagy and Cancer Therapy
Abstract
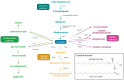
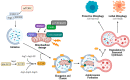
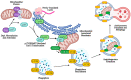
Sphingolipids are bioactive lipids responsible for regulating diverse cellular functions such as proliferation, migration, senescence, and death. These lipids are characterized by a long-chain sphingosine backbone amide-linked to a fatty acyl chain with variable length. The length of the fatty acyl chain is determined by specific ceramide synthases, and this fatty acyl length also determines the sphingolipid's specialized functions within the cell. One function in particular, the regulation of the selective autophagy of mitochondria, or mitophagy, is closely regulated by ceramide, a key regulatory sphingolipid. Mitophagy alterations have important implications for cancer cell proliferation, response to chemotherapeutics, and mitophagy-mediated cell death. This review will focus on the alterations of ceramide synthases in cancer and sphingolipid regulation of lethal mitophagy, concerning cancer therapy.
Keywords: apoptosis; cancer; ceramide; mitophagy; sphingolipids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ward E.M., Sherman R.L., Henley S.J., Jemal A., Siegel D.A., Feuer E.J., Firth A.U., Kohler B.A., Scott S., Ma J., et al. Annual report to the nation on the status of cancer, featuring cancer in men and women age 20–49 years. JNCI J. Natl. Cancer Inst. 2019;111:1279–1297. doi: 10.1093/jnci/djz106. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

