Multimodal Imaging Techniques to Evaluate the Anticancer Effect of Cold Atmospheric Pressure Plasma
- PMID: 34069689
- PMCID: PMC8161248
- DOI: 10.3390/cancers13102483
Multimodal Imaging Techniques to Evaluate the Anticancer Effect of Cold Atmospheric Pressure Plasma
Abstract
Background: Skin cancer is the most frequent cancer worldwide and is divided into non-melanoma skin cancer, including basal cell carcinoma, as well as squamous cell carcinoma (SCC) and malignant melanoma (MM).
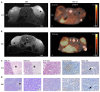
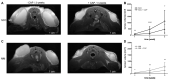
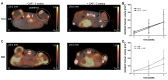
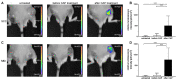
Methods: This study evaluates the effects of cold atmospheric pressure plasma (CAP) on SCC and MM in vivo, employing a comprehensive approach using multimodal imaging techniques. Longitudinal MR and PET/CT imaging were performed to determine the anatomic and metabolic tumour volume over three-weeks in vivo. Additionally, the formation of reactive species after CAP treatment was assessed by non-invasive chemiluminescence imaging of L-012. Histological analysis and immunohistochemical staining for Ki-67, ApopTag®, F4/80, CAE, and CD31, as well as protein expression of PCNA, caspase-3 and cleaved-caspase-3, were performed to study proliferation, apoptosis, inflammation, and angiogenesis in CAP-treated tumours.
Results: As the main result, multimodal in vivo imaging revealed a substantial reduction in tumour growth and an increase in reactive species after CAP treatment, in comparison to untreated tumours. In contrast, neither the markers for apoptosis, nor the metabolic activity of both tumour entities was affected by CAP.
Conclusions: These findings propose CAP as a potential adjuvant therapy option to established standard therapies of skin cancer.
Keywords: kINPen™; malignant melanoma; plasma medicine; reactive oxygen and nitrogen species; skin cancer; squamous cell carcinoma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Cold atmospheric pressure plasma treatment combined with starvation increases autophagy and apoptosis in melanoma in vitro and in vivo.Exp Dermatol. 2022 Jul;31(7):1016-1028. doi: 10.1111/exd.14544. Epub 2022 Feb 26. Exp Dermatol. 2022. PMID: 35181947
-
Differential Sensitivity of Melanoma Cells and Their Non-Cancerous Counterpart to Cold Atmospheric Plasma-Induced Reactive Oxygen and Nitrogen Species.Int J Mol Sci. 2022 Nov 15;23(22):14092. doi: 10.3390/ijms232214092. Int J Mol Sci. 2022. PMID: 36430569 Free PMC article.
-
Use of Cold Atmospheric Plasma in the Treatment of Squamous Cell Carcinoma: in vitro Effects and Clinical Application in Feline Tumors: A Pilot Study.Top Companion Anim Med. 2023 Mar-Jun;53-54:100773. doi: 10.1016/j.tcam.2023.100773. Epub 2023 Mar 27. Top Companion Anim Med. 2023. PMID: 36990177
-
Recent advances in cold atmospheric plasma (CAP) for breast cancer therapy.Cell Biol Int. 2023 Feb;47(2):327-340. doi: 10.1002/cbin.11939. Epub 2022 Nov 7. Cell Biol Int. 2023. PMID: 36342241 Review.
-
Molecular Mechanisms of the Efficacy of Cold Atmospheric Pressure Plasma (CAP) in Cancer Treatment.Cancers (Basel). 2020 Jan 22;12(2):269. doi: 10.3390/cancers12020269. Cancers (Basel). 2020. PMID: 31979114 Free PMC article. Review.
Cited by
-
Patient-Derived Human Basal and Cutaneous Squamous Cell Carcinoma Tissues Display Apoptosis and Immunomodulation following Gas Plasma Exposure with a Certified Argon Jet.Int J Mol Sci. 2021 Oct 23;22(21):11446. doi: 10.3390/ijms222111446. Int J Mol Sci. 2021. PMID: 34768877 Free PMC article.
-
Modeling Gas Plasma-Tissue Interactions in 3D Collagen-Based Hydrogel Cancer Cell Cultures.Bioengineering (Basel). 2023 Mar 17;10(3):367. doi: 10.3390/bioengineering10030367. Bioengineering (Basel). 2023. PMID: 36978758 Free PMC article.
-
Modulation of the Tumor-Associated Immuno-Environment by Non-Invasive Physical Plasma.Cancers (Basel). 2023 Feb 8;15(4):1073. doi: 10.3390/cancers15041073. Cancers (Basel). 2023. PMID: 36831415 Free PMC article. Review.
-
Current Status and Future Trends of Cold Atmospheric Plasma as an Oncotherapy.Biomol Ther (Seoul). 2023 Sep 1;31(5):496-514. doi: 10.4062/biomolther.2023.027. Biomol Ther (Seoul). 2023. PMID: 37641880 Free PMC article. Review.
-
Medical gas plasma technology: Roadmap on cancer treatment and immunotherapy.Redox Biol. 2023 Sep;65:102798. doi: 10.1016/j.redox.2023.102798. Epub 2023 Jun 27. Redox Biol. 2023. PMID: 37556976 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

