A 3D Renal Proximal Tubule on Chip Model Phenocopies Lowe Syndrome and Dent II Disease Tubulopathy
- PMID: 34069732
- PMCID: PMC8161077
- DOI: 10.3390/ijms22105361
A 3D Renal Proximal Tubule on Chip Model Phenocopies Lowe Syndrome and Dent II Disease Tubulopathy
Abstract
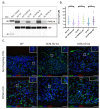
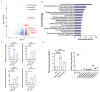
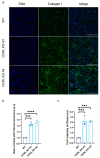
Lowe syndrome and Dent II disease are X-linked monogenetic diseases characterised by a renal reabsorption defect in the proximal tubules and caused by mutations in the OCRL gene, which codes for an inositol-5-phosphatase. The life expectancy of patients suffering from Lowe syndrome is largely reduced because of the development of chronic kidney disease and related complications. There is a need for physiological human in vitro models for Lowe syndrome/Dent II disease to study the underpinning disease mechanisms and to identify and characterise potential drugs and drug targets. Here, we describe a proximal tubule organ on chip model combining a 3D tubule architecture with fluid flow shear stress that phenocopies hallmarks of Lowe syndrome/Dent II disease. We demonstrate the high suitability of our in vitro model for drug target validation. Furthermore, using this model, we demonstrate that proximal tubule cells lacking OCRL expression upregulate markers typical for epithelial-mesenchymal transition (EMT), including the transcription factor SNAI2/Slug, and show increased collagen expression and deposition, which potentially contributes to interstitial fibrosis and disease progression as observed in Lowe syndrome and Dent II disease.
Keywords: Lowe syndrome; OCRL; disease modeling; fibrosis; microfluidic; organ-on-a-chip; proximal tubule-on-a-chip.
Conflict of interest statement
H.L.L. is an employee of Mimetas BV, which markets the OrganoPlate. OrganoPlate is a registered trademark of Mimetas BV. The authors have no additional financial interests.
Figures





Similar articles
-
OCRL-mutated fibroblasts from patients with Dent-2 disease exhibit INPP5B-independent phenotypic variability relatively to Lowe syndrome cells.Hum Mol Genet. 2015 Feb 15;24(4):994-1006. doi: 10.1093/hmg/ddu514. Epub 2014 Oct 9. Hum Mol Genet. 2015. PMID: 25305077
-
OCRL deficiency impairs endolysosomal function in a humanized mouse model for Lowe syndrome and Dent disease.Hum Mol Genet. 2019 Jun 15;28(12):1931-1946. doi: 10.1093/hmg/ddy449. Hum Mol Genet. 2019. PMID: 30590522 Free PMC article.
-
Kidney Tubular Ablation of Ocrl/Inpp5b Phenocopies Lowe Syndrome Tubulopathy.J Am Soc Nephrol. 2017 May;28(5):1399-1407. doi: 10.1681/ASN.2016080913. Epub 2016 Nov 28. J Am Soc Nephrol. 2017. PMID: 27895154 Free PMC article.
-
The cellular and physiological functions of the Lowe syndrome protein OCRL1.Traffic. 2014 May;15(5):471-87. doi: 10.1111/tra.12160. Epub 2014 Mar 7. Traffic. 2014. PMID: 24499450 Free PMC article. Review.
-
The 5-phosphatase OCRL in Lowe syndrome and Dent disease 2.Nat Rev Nephrol. 2017 Aug;13(8):455-470. doi: 10.1038/nrneph.2017.83. Epub 2017 Jul 3. Nat Rev Nephrol. 2017. PMID: 28669993 Review.
Cited by
-
Organs-on-chip technology: a tool to tackle genetic kidney diseases.Pediatr Nephrol. 2022 Dec;37(12):2985-2996. doi: 10.1007/s00467-022-05508-2. Epub 2022 Mar 14. Pediatr Nephrol. 2022. PMID: 35286457 Free PMC article. Review.
-
Organoids-on-Chips Technology: Unveiling New Perspectives in Rare-Disease Research.Int J Mol Sci. 2025 May 4;26(9):4367. doi: 10.3390/ijms26094367. Int J Mol Sci. 2025. PMID: 40362604 Free PMC article. Review.
-
A systematic review of kidney-on-a-chip-based models to study human renal (patho-)physiology.Dis Model Mech. 2023 Jun 1;16(6):dmm050113. doi: 10.1242/dmm.050113. Epub 2023 Jun 19. Dis Model Mech. 2023. PMID: 37334839 Free PMC article.
-
Microfluidic Systems to Mimic the Blood-Brain Barrier: from Market to Engineering Challenges and Perspectives.ACS Biomater Sci Eng. 2025 Jul 14;11(7):3789-3815. doi: 10.1021/acsbiomaterials.4c02221. Epub 2025 Jun 25. ACS Biomater Sci Eng. 2025. PMID: 40560145 Free PMC article. Review.
-
Analysis of reproducibility and robustness of a renal proximal tubule microphysiological system OrganoPlate 3-lane 40 for in vitro studies of drug transport and toxicity.Toxicol Sci. 2023 Oct 30;196(1):52-70. doi: 10.1093/toxsci/kfad080. Toxicol Sci. 2023. PMID: 37555834 Free PMC article.
References
-
- Zaniew M., Bökenkamp A., Niemirska A., Bürger J., Rogowska-Kalisz A., Gellermann J., Zampetoglou A., Roszak M., Moczko J., Krzemień A., et al. Long-term renal outcome in children withOCRLmutations: Retrospective analysis of a large international cohort. Nephrol. Dial. Transplant. 2016;33:85–94. doi: 10.1093/ndt/gfw350. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

