Study on the Corrosion Resistance of Graphene Oxide-Based Epoxy Zinc-Rich Coatings
- PMID: 34069742
- PMCID: PMC8160921
- DOI: 10.3390/polym13101657
Study on the Corrosion Resistance of Graphene Oxide-Based Epoxy Zinc-Rich Coatings
Abstract
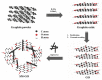
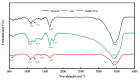
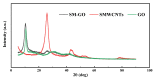
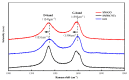
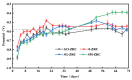
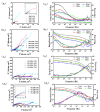
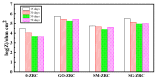
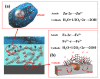
In order to improve the corrosion resistance of zinc-rich epoxy coatings and reduce the amount of zinc used, first, graphene oxide (GO) was modified by sulfonated multiwall carbon nanotubes (SMWCNTs) to obtain the modified graphene oxide (SM-GO). The samples were characterized by Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD) and Raman spectroscopy. Then, four kinds of coatings were prepared, namely pure zinc-rich coating (0-ZRC), graphene oxide-based zinc-rich coating (GO-ZRC), sulfonated multiwall carbon nanotube-based zinc-rich coating (SM-ZRC) and SM-GO-based zinc-rich coating (SG-ZRC). The corrosion resistance of the above coatings was studied by open circuit potential (OCP), electrochemical impedance spectroscopy (EIS), a salt spray test, 3D confocal microscope, and electron scanning electron microscope (SEM). The results indicate that GO is successfully non-covalently modified by SMWCNTs, of which the interlayer spacing increases and dispersion is improved. The order of the corrosion resistance is GO-ZRC > SG-ZRC > SM-ZRC > 0-ZRC. The addition of GO, SMWCNTs, and SM-GO increases the shielding effect and increases the electrical connection between Zn particles and metal substrates, which improves the corrosion resistance. However, SMWCNTs and SM-GO also strengthen the galvanic corrosion, which decreases the corrosion resistance to some extent.
Keywords: corrosion resistance; epoxy zinc-rich coating; graphene oxide; multiwall carbon nanotubes; sulfonated.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Incorporation of Graphene Oxide Modified with Polyamide Curing Agent into the Epoxy-Zinc Composite Coating for Promoting Its Corrosion Resistance.Polymers (Basel). 2023 Apr 13;15(8):1873. doi: 10.3390/polym15081873. Polymers (Basel). 2023. PMID: 37112020 Free PMC article.
-
GO-Ti3C2 two-dimensional heterojunction nanomaterial for anticorrosion enhancement of epoxy zinc-rich coatings.J Hazard Mater. 2021 Sep 5;417:126048. doi: 10.1016/j.jhazmat.2021.126048. Epub 2021 May 7. J Hazard Mater. 2021. PMID: 33992004
-
Designed a novel EP + GO/ZRC + GO coating with bilayered structure for enhancing corrosion resistance of steel substrate.J Hazard Mater. 2021 Feb 5;403:123670. doi: 10.1016/j.jhazmat.2020.123670. Epub 2020 Aug 13. J Hazard Mater. 2021. PMID: 33264874
-
Graphene-Based Impregnation into Polymeric Coating for Corrosion Resistance.Nanomaterials (Basel). 2025 Mar 24;15(7):486. doi: 10.3390/nano15070486. Nanomaterials (Basel). 2025. PMID: 40214532 Free PMC article. Review.
-
Recent Advances in Corrosion Inhibition of Bonded NdFeB Magnets.Materials (Basel). 2024 May 21;17(11):2475. doi: 10.3390/ma17112475. Materials (Basel). 2024. PMID: 38893739 Free PMC article. Review.
Cited by
-
Experimental Investigations of AlMg3 Components with Polyurethane and Graphene Oxide Nanosheets Composite Coatings, after Accelerated UV-Aging.Molecules. 2021 Dec 23;27(1):84. doi: 10.3390/molecules27010084. Molecules. 2021. PMID: 35011316 Free PMC article.
-
Study on the Effect of Deposited Graphene Oxide on the Fatigue Life of Austenitic Steel 1.4541 in Different Temperature Ranges.Materials (Basel). 2021 Dec 22;15(1):65. doi: 10.3390/ma15010065. Materials (Basel). 2021. PMID: 35009211 Free PMC article.
-
Incorporation of Graphene Oxide Modified with Polyamide Curing Agent into the Epoxy-Zinc Composite Coating for Promoting Its Corrosion Resistance.Polymers (Basel). 2023 Apr 13;15(8):1873. doi: 10.3390/polym15081873. Polymers (Basel). 2023. PMID: 37112020 Free PMC article.
-
Incorporating graphene-modified mica and conductive nickel particles for enhanced corrosion resistance in epoxy zinc-rich coatings.Front Chem. 2025 Apr 30;13:1544762. doi: 10.3389/fchem.2025.1544762. eCollection 2025. Front Chem. 2025. PMID: 40370413 Free PMC article.
-
Synergistic Effect of CNT and N-Doped Graphene Foam on Improving the Corrosion Resistance of Zn Reinforced Epoxy Composite Coatings.Polymers (Basel). 2024 Dec 17;16(24):3513. doi: 10.3390/polym16243513. Polymers (Basel). 2024. PMID: 39771365 Free PMC article.
References
-
- Marchebois H., Savall C., Bernard J., Touzain S. Electrochemical behavior of zinc-rich powder coatings in artificial sea water. Electrochim. Acta. 2004;49:2945–2954. doi: 10.1016/j.electacta.2004.01.053. - DOI
-
- Zhou S., Wu Y., Zhao W., Yu J., Jiang F., Wu Y., Ma L. Designing reduced graphene oxide/zinc rich epoxy composite coatings for improving the anticorrosion performance of carbon steel substrate. Mater. Des. 2019;169:107694. doi: 10.1016/j.matdes.2019.107694. - DOI
-
- Teng S., Gao Y., Cao F., Kong D., Zheng X., Ma X., Zhi L. Zinc-reduced graphene oxide for enhanced corrosion protection of zinc-rich epoxy coatings. Prog. Org. Coat. 2018;123:185–189. doi: 10.1016/j.porgcoat.2018.07.012. - DOI
-
- Shirehjini F.T., Danaee I., Eskandari H., Zarei D. Effect of Nano Clay on Corrosion Protection of Zinc-rich Epoxy Coatings on Steel 37. J. Mater. Sci. Technol. 2016;32:1152–1160. doi: 10.1016/j.jmst.2016.08.017. - DOI
-
- Ding R., Zheng Y., Yu H., Li W., Wang X., Gui T. Study of water permeation dynamics and anti-corrosion mechanism of graphene/zinc coatings. J. Alloy. Compd. 2018;748:481–495. doi: 10.1016/j.jallcom.2018.03.160. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

