BPA, BPAF and TMBPF Alter Adipogenesis and Fat Accumulation in Human Mesenchymal Stem Cells, with Implications for Obesity
- PMID: 34069744
- PMCID: PMC8160667
- DOI: 10.3390/ijms22105363
BPA, BPAF and TMBPF Alter Adipogenesis and Fat Accumulation in Human Mesenchymal Stem Cells, with Implications for Obesity
Abstract
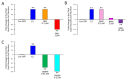
Bisphenol A (BPA) is an endocrine-disrupting chemical used in the production of plastics, and is linked to developmental, reproductive, and metabolic disorders including obesity. Manufacturers have begun using 'BPA-free' alternatives instead of BPA in many consumer products. However, these alternatives have had much less testing and oversight, yet they are already being mass-produced and used across industries from plastics to food-contact coatings. Here, we used human female adipose-derived stem cells (hASCs), a type of adult mesenchymal stem cell, to compare the effects of BPA and BPA alternatives on adipogenesis or fat cell development in vitro. We focused on two commonly used BPA replacements, bisphenol AF (BPAF) and tetramethyl bisphenol F (TMBPF; monomer of the new valPure V70 food-contact coating). Human ASCs were differentiated into adipocytes using chemically defined media in the presence of control differentiation media with and without 17β-estradiol (E2; 10 μM), or with increasing doses of BPA (0, 0.1 and 1 μM), BPAF (0, 0.1, 1 and 10 nM), or TMBPF (0, 0.01 and 0.1 μM). After differentiation, the cells were stained and imaged to visualize and quantify the accumulation of lipid vacuoles and number of developing fat cells. Treated cells were also examined for cell viability and apoptosis (programmed cell death) using the respective cellular assays. Similar to E2, BPA at 0.1 μM and BPAF at 0.1 nM, significantly increased adipogenesis and lipid production by 20% compared to control differentiated cells (based on total lipid vacuole number to cell number ratios), whereas higher levels of BPA and BPAF significantly decreased adipogenesis (p < 0.005). All tested doses of TMBPF significantly reduced adipogenesis and lipid production by 30-40%, likely at least partially through toxic effects on stem cells, as viable cell numbers decreased and apoptosis levels increased throughout differentiation. These findings indicate that low, environmentally-relevant doses of BPA, BPAF, and TMBPF have significant effects on fat cell development and lipid accumulation, with TMBPF having non-estrogenic, anti-adipogenic effects. These and other recent results may provide a potential cellular mechanism between exposure to bisphenols and human obesity, and underscore the likely impact of these chemicals on fat development in vivo.
Keywords: BPA; BPAF; TMBPF; adipogenesis; adipose-derived stem cells (ASCs); endocrine-disrupting chemicals (EDCs); food-contact coating; obesity; plastics; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Onundi Y., Drake B.A., Malecky R.T., DeNardo M.A., Mills M.R., Kundu S., Ryabov A.D., Beach E.S., Horwitz C.P., Simonich M.T., et al. A multidisciplinary investigation of the technical and environmental performances of TAML/peroxide elimination of Bisphenol A compounds from water. Green Chem. 2017;19:4234–4262. doi: 10.1039/C7GC01415E. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

