Sexual Dimorphism of Corticosteroid Signaling during Kidney Development
- PMID: 34069759
- PMCID: PMC8155845
- DOI: 10.3390/ijms22105275
Sexual Dimorphism of Corticosteroid Signaling during Kidney Development
Abstract
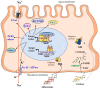
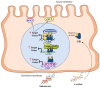
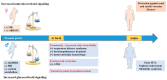
Sexual dimorphism involves differences between biological sexes that go beyond sexual characteristics. In mammals, differences between sexes have been demonstrated regarding various biological processes, including blood pressure and predisposition to develop hypertension early in adulthood, which may rely on early events during development and in the neonatal period. Recent studies suggest that corticosteroid signaling pathways (comprising glucocorticoid and mineralocorticoid signaling pathways) have distinct tissue-specific expression and regulation during this specific temporal window in a sex-dependent manner, most notably in the kidney. This review outlines the evidence for a gender differential expression and activation of renal corticosteroid signaling pathways in the mammalian fetus and neonate, from mouse to human, that may favor mineralocorticoid signaling in females and glucocorticoid signaling in males. Determining the effects of such differences may shed light on short term and long term pathophysiological consequences, markedly for males.
Keywords: aldosterone; cortisol; development; kidney; mineralocorticoid and glucocorticoid receptors; neonates; sexual dimorphism.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

