The Impact of Pneumococcal Conjugate Vaccine (PCV) Coverage Heterogeneities on the Changing Epidemiology of Invasive Pneumococcal Disease in Switzerland, 2005-2019
- PMID: 34069761
- PMCID: PMC8157260
- DOI: 10.3390/microorganisms9051078
The Impact of Pneumococcal Conjugate Vaccine (PCV) Coverage Heterogeneities on the Changing Epidemiology of Invasive Pneumococcal Disease in Switzerland, 2005-2019
Abstract
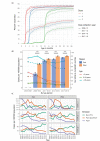
Pneumococcal conjugate vaccines (PCVs) have lowered the incidence of invasive pneumococcal disease (IPD) worldwide. However, the influence of regional vaccine uptake differences on the changing epidemiology of IPD remains unclear. We aimed to examine the overall impact of both seven- and 13-valent PCVs (PCV7 and PCV13) on IPD in Switzerland. Three-year periods from 2005-2010 and 2011-2019 were considered, respectively, as (early and late) PCV7 eras and (early, mid and late) PCV13 eras. Vaccine coverage was estimated from a nationwide survey according to east (German-speaking) and west (French/Italian-speaking) regions for each period. Reported incidence rate ratios (IRRs) were compared between successive periods and regions using nationwide IPD surveillance data. Overall IPD incidence across all ages was only 16% lower in the late PCV13 era compared to the early PCV7 era (IRR 0.83, 95% CI 0.79-0.88), due to increasing incidence of non-PCV-type IPD (2.59, 2.37-2.83) in all age groups, except children <5 years. PCV uptake rates in swiss children were slightly higher in the west than the east (p < 0.001), and were accompanied by lower IPD incidences across all age groups in the former region. Post-PCV13, non-PCV serotypes 8, 22F and 9N were the major cause of IPD in adults ≥65 years. Increased PCV coverage in both areas of Switzerland resulted in a decrease in vaccine-type and overall IPD incidence across all age groups, in a regionally dependent manner. However, the rising incidence of non-vaccine-type IPD, exclusive to older adults, may undermine indirect beneficial effects.
Keywords: Streptococcus pneumoniae; Switzerland; capsular polysaccharide; pneumococcal conjugate vaccines (PCVs); serotype 3.
Conflict of interest statement
M.H. received an educational grant from Pfizer AG. W.C.A. and M.H. served on an advisory board for MSD, for which reimbursement was paid to his institution. The funders had no role in the design of the study; data collection, analyses, or interpretation; nor in the writing of the manuscript, or the decision to publish the results.
Figures



References
-
- WHO Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly. Epidemiol. Rec. 2007;82:93–104. - PubMed
-
- Ciruela P., Izquierdo C., Broner S., Muñoz-Almagro C., Hernández S., Ardanuy C., Pallarés R., Domínguez A., Jané M., Esteva C., et al. The changing epidemiology of invasive pneumococcal disease after PCV13 vaccination in a country with intermediate vaccination coverage. Vaccine. 2018;36:7744–7752. doi: 10.1016/j.vaccine.2018.05.026. - DOI - PubMed
-
- Hays C., Vermee Q., Agathine A., Dupuis A., Varon E., Poyart C., Ploy M.-C., Raymond J. Demonstration of the herd effect in adults after the implementation of pneumococcal vaccination with PCV13 in children. Eur. J. Clin. Microbiol. Infect. Dis. 2016;36:831–838. doi: 10.1007/s10096-016-2868-5. - DOI - PubMed
-
- Bundesamt für Gesundheit. Eidgenössische Kommission für Impffragen . Empfehlungen zur Pneumokokkenimpfung bei Kindern unter 5 Jahren. Richtlinien und Empfehlungen (ehemals Supplementum XVII) Bundesamt für Gesundheit; Liebefeld, Switzerland: 2007. pp. 1–16.
-
- Bundesamt für Gesundheit. Eidgenössische Kommission für Impffragen Empfehlungen zur Pneumokokkenimpfung bei Kindern unter 5 Jahren: Wechsel vom 7- zum 13-valenten konjugierten Impfstoff. Bull. BAG. 2010;51:1202–1205.
Grants and funding
LinkOut - more resources
Full Text Sources

