Mitochondrial Fission Governed by Drp1 Regulates Exogenous Fatty Acid Usage and Storage in Hela Cells
- PMID: 34069800
- PMCID: PMC8157282
- DOI: 10.3390/metabo11050322
Mitochondrial Fission Governed by Drp1 Regulates Exogenous Fatty Acid Usage and Storage in Hela Cells
Abstract
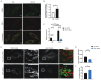
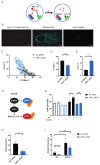
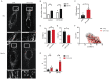
In the presence of high abundance of exogenous fatty acids, cells either store fatty acids in lipid droplets or oxidize them in mitochondria. In this study, we aimed to explore a novel and direct role of mitochondrial fission in lipid homeostasis in HeLa cells. We observed the association between mitochondrial morphology and lipid droplet accumulation in response to high exogenous fatty acids. We inhibited mitochondrial fission by silencing dynamin-related protein 1(DRP1) and observed the shift in fatty acid storage-usage balance. Inhibition of mitochondrial fission resulted in an increase in fatty acid content of lipid droplets and a decrease in mitochondrial fatty acid oxidation. Next, we overexpressed carnitine palmitoyltransferase-1 (CPT1), a key mitochondrial protein in fatty acid oxidation, to further examine the relationship between mitochondrial fatty acid usage and mitochondrial morphology. Mitochondrial fission plays a role in distributing exogenous fatty acids. CPT1A controlled the respiratory rate of mitochondrial fatty acid oxidation but did not cause a shift in the distribution of fatty acids between mitochondria and lipid droplets. Our data reveals a novel function for mitochondrial fission in balancing exogenous fatty acids between usage and storage, assigning a role for mitochondrial dynamics in control of intracellular fuel utilization and partitioning.
Keywords: fatty acid oxidation; lipid homeostasis; mitochondrial dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Buck M.D., Sullivan D.O., Geltink R.I.K., Curtis J.D., Chang C.-H., Sanin D.E., Qiu J., Kretz O., Braas D., Chen Q., et al. Mitochondrial Dynamics Controls T Cell Fate through Article Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell. 2016;166:1–14. doi: 10.1016/j.cell.2016.05.035. - DOI - PMC - PubMed
Grants and funding
- NKFI-KPP-126998/Hungarian National Research, Development and Innovation Office
- R01 DK110181/DK/NIDDK NIH HHS/United States
- R01 DK127637/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- AG052005, AG052986, AG051459, DK111178, DK045735, DK097566, DK110181, DK045735-26/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

