Computational Treatment Simulations to Assess the Need for Personalized Tamoxifen Dosing in Breast Cancer Patients of Different Biogeographical Groups
- PMID: 34069810
- PMCID: PMC8157244
- DOI: 10.3390/cancers13102432
Computational Treatment Simulations to Assess the Need for Personalized Tamoxifen Dosing in Breast Cancer Patients of Different Biogeographical Groups
Abstract
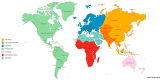
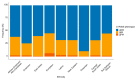
Tamoxifen is used worldwide to treat estrogen receptor-positive breast cancer. It is extensively metabolized, and minimum steady-state concentrations of its metabolite endoxifen (CSS,min ENDX) >5.97 ng/mL have been associated with favorable outcome. Endoxifen formation is mediated by the enzyme CYP2D6, and impaired CYP2D6 function has been associated with lower CSS,min ENDX. In the Women's Healthy Eating and Living (WHEL) study proposing the target concentration, 20% of patients showed subtarget CSS,min ENDX at tamoxifen standard dosing. CYP2D6 allele frequencies vary largely between populations, and as 87% of the patients in the WHEL study were White, little is known about the risk for subtarget CSS,min ENDX in other populations. Applying pharmacokinetic simulations, this study investigated the risk for subtarget CSS,min ENDX at tamoxifen standard dosing and the need for dose individualization in nine different biogeographical groups with distinct CYP2D6 allele frequencies. The high variability in CYP2D6 allele frequencies amongst the biogeographical groups resulted in an up to three-fold difference in the percentages of patients with subtarget CSS,min ENDX. Based on their CYP2D6 allele frequencies, East Asian breast cancer patients were identified as the population for which personalized, model-informed precision dosing would be most beneficial (28% of patients with subtarget CSS,min ENDX).
Keywords: CYP2D6; breast cancer; genotype; individualized dosing; model-informed precision dosing; personalized dosing; polymorphism; tamoxifen.
Conflict of interest statement
C.K. and W.H. report grants from an industry consortium (AbbVie Deutschland GmbH & Co. KG, AstraZeneca Ltd., Boehringer Ingelheim Pharma GmbH & Co. KG, Grünenthal GmbH, F. Hoffmann-La Roche Ltd., Merck KGaA and Sanofi) for the PharMetrX PhD program. C.K. reports grants for the Innovative Medicines Initiative-Joint Undertaking (‘DDMoRe’), Diurnal Ltd., from the Federal Ministry of Education and Research as well as the European Commission, all outside the submitted work. L.K.S. is a current employee of Merck Healthcare KGaA. M.S. reports grants from Gilead Sciences Inc. (Foster, CA, USA), Green Cross WellBeing Co. Ltd., Agena Bioscience GmbH, and honoraria for oral presentations at academically organized congresses and meetings. All other authors declared no competing interest for this work.
Figures

References
-
- Abe O., Abe R., Enomoto K., Kikuchi K., Koyama H., Masuda H., Nomura Y., Ohashi Y., Sakai K., Sugimachi K., et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/s0140-6736(11)60993-8. - DOI - PMC - PubMed
-
- Burstein H.J., Lacchetti C., Anderson H., Buchholz T.A., Davidson N.E., Gelmon K.A., Giordano S.H., Hudis C.A., Solky A.J., Stearns V., et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor–Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2019;37:423–438. doi: 10.1200/JCO.18.01160. - DOI - PubMed
-
- Johnson M.D., Zuo H., Lee K.-H., Trebley J.P., Rae J.M., Weatherman R.V., Desta Z., Flockhart D.A., Skaar T.C. Pharmacological Characterization of 4-hydroxy-N-desmethyl Tamoxifen, a Novel Active Metabolite of Tamoxifen. Breast Cancer Res. Treat. 2004;85:151–159. doi: 10.1023/B:BREA.0000025406.31193.e8. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

