Paternal High-Protein Diet Programs Offspring Insulin Sensitivity in a Sex-Specific Manner
- PMID: 34069853
- PMCID: PMC8157381
- DOI: 10.3390/biom11050751
Paternal High-Protein Diet Programs Offspring Insulin Sensitivity in a Sex-Specific Manner
Abstract
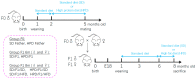
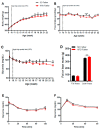
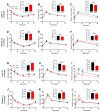
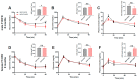
The impact of maternal nutrition on offspring is well documented. However, the implication of pre-conceptional paternal nutrition on the metabolic health of the progeny remains underexplored. Here, we investigated the impact of paternal high-protein diet (HPD, 43.2% protein) consumption on the endocrine pancreas and the metabolic phenotype of offspring. Male Wistar rats were given HPD or standard diet (SD, 18.9% protein) for two months. The progenies (F1) were studied at fetal stage and in adulthood. Body weight, glycemia, glucose tolerance (GT), glucose-induced insulin secretion in vivo (GIIS) and whole-body insulin sensitivity were assessed in male and female F1 offspring. Insulin sensitivity, GT and GIIS were similar between F1 females from HPD (HPD/F1) and SD fathers (SD/F1). Conversely, male HPD/F1 exhibited increased insulin sensitivity (p < 0.05) and decreased GIIS (p < 0.05) compared to male SD/F1. The improvement of insulin sensitivity in HPD/F1 was sustained even after 2 months of high-fat feeding. In male HPD/F1, the β cell mass was preserved and the β cell plasticity, following metabolic challenge, was enhanced compared to SD/F1. In conclusion, we provide the first evidence of a sex-specific impact of paternal HPD on the insulin sensitivity and GIIS of their descendants, demonstrating that changes in paternal nutrition alter the metabolic status of their progeny in adulthood.
Keywords: endocrine pancreas; glucose homeostasis; high-protein diet; insulin secretion; insulin sensitivity; paternal programming; sperm small non-coding RNAs.
Conflict of interest statement
The authors declare no conflict of interest associated with this manuscript.
Figures





References
-
- Dunford A.R., Sangster J.M. Maternal and Paternal Periconceptional Nutrition as an Indicator of Offspring Metabolic Syndrome Risk in Later Life through Epigenetic Imprinting: A Systematic Review. Diabetes Metab. Syndr. Clin. Res. Rev. 2017;11:S655–S662. doi: 10.1016/j.dsx.2017.04.021. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

