Proton Detected Solid-State NMR of Membrane Proteins at 28 Tesla (1.2 GHz) and 100 kHz Magic-Angle Spinning
- PMID: 34069858
- PMCID: PMC8157399
- DOI: 10.3390/biom11050752
Proton Detected Solid-State NMR of Membrane Proteins at 28 Tesla (1.2 GHz) and 100 kHz Magic-Angle Spinning
Abstract
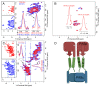
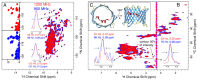
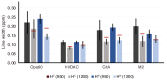
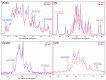
The available magnetic field strength for high resolution NMR in persistent superconducting magnets has recently improved from 23.5 to 28 Tesla, increasing the proton resonance frequency from 1 to 1.2 GHz. For magic-angle spinning (MAS) NMR, this is expected to improve resolution, provided the sample preparation results in homogeneous broadening. We compare two-dimensional (2D) proton detected MAS NMR spectra of four membrane proteins at 950 and 1200 MHz. We find a consistent improvement in resolution that scales superlinearly with the increase in magnetic field for three of the four examples. In 3D and 4D spectra, which are now routinely acquired, this improvement indicates the ability to resolve at least 2 and 2.5 times as many signals, respectively.
Keywords: beta barrel; high magnetic field; magic-angle spinning; membrane protein; proton detection; solid-state NMR; transmembrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bodenhausen G., Ruben D.J. Natural abundance nitrogen-15 NMR by enhanced heteronuclear spectroscopy. Chem. Phys. Lett. 1980;69:185–189. doi: 10.1016/0009-2614(80)80041-8. - DOI
-
- Maricq M.M., Waugh J.S. NMR in rotating solids. J. Chem. Phys. 1979;70:3300–3316. doi: 10.1063/1.437915. - DOI
-
- Chevelkov V., van Rossum B.J., Castellani F., Rehbein K., Diehl A., Hohwy M., Steuernagel S., Engelke F., Oschkinat H., Reif B. 1H detection in MAS solid-state NMR spectroscopy of biomacromolecules employing pulsed field gradients for residual solvent suppression. J. Am. Chem. Soc. 2003;125:7788–7789. doi: 10.1021/ja029354b. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

