Cytokine-Mediated Inflammation in the Oral Cavity and Its Effect on Lipid Nanocarriers
- PMID: 34070004
- PMCID: PMC8157841
- DOI: 10.3390/nano11051330
Cytokine-Mediated Inflammation in the Oral Cavity and Its Effect on Lipid Nanocarriers
Abstract
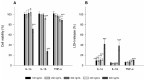
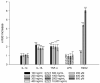
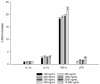
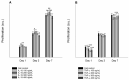
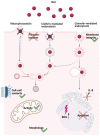
Topical drug administration to the oral mucosa proves to be a promising treatment alternative for inflammatory diseases. However, disease-related changes in the cell barrier must be considered when developing such delivery systems. This study aimed at investigating the changes in the lining mucosa caused by inflammation and evaluating the consequences on drug delivery systems such as nanostructured lipid carriers (NLC). For this, TR146 cells were treated with inflammatory cytokines and bacterial components. Cell viability and integrity, reactive oxygen species (ROS), and interleukin (IL)-8 release were used as endpoints to assess inflammation. Translocation of phosphatidylserine, cytoskeletal arrangement, opening of desmosomes, and cell proliferation were examined. Transport studies with NLC were performed considering active and passive pathways. The results showed that IL-1ß and tumor necrosis factor α induced inflammation by increasing IL-8 and ROS production (22-fold and 2-fold). Morphologically, loss of cell-cell connections and formation of stress fibers and hyperplasia were observed. The charge of the cell membrane shifted from neutral to negative, which increased the absorption of NLC due to the repulsive interactions between the hydrophobic negative particles and the cell membrane on the one hand, and interactions with lipophilic membrane proteins such as caveolin on the other.
Keywords: TR146; cytokine; in-vitro inflammation; lining mucosa; nanostructured lipid carriers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- World Health Organization Oral Health. [(accessed on 6 November 2019)]; Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health.
-
- Platform for Better Oral Health in Europe The State of Oral Health in Europe. [(accessed on 6 November 2019)]; Available online: http://www.oralhealthplatform.eu/our-work/the-state-of-oral-health-in-eu...
LinkOut - more resources
Full Text Sources

