Influence of Serotonin 5-HT4 Receptors on Responses to Cardiac Stressors in Transgenic Mouse Models
- PMID: 34070090
- PMCID: PMC8158346
- DOI: 10.3390/biomedicines9050569
Influence of Serotonin 5-HT4 Receptors on Responses to Cardiac Stressors in Transgenic Mouse Models
Abstract
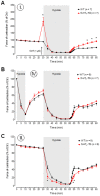
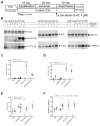
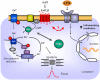
The current study aimed to deepen our knowledge on the role of cardiac 5-HT4 receptors under pathophysiological conditions. To this end, we used transgenic (TG) mice that overexpressed human 5-HT4a receptors solely in cardiac myocytes (5-HT4-TG mice) and their wild-type (WT) littermates that do not have functional cardiac 5-HT4 receptors as controls. We found that an inflammation induced by lipopolysaccharide (LPS) was detrimental to cardiac function in both 5-HT4-TG and WT mice. In a hypoxia model, isolated left atrial preparations from the 5-HT4-TG mice went into contracture faster during hypoxia and recovered slower following hypoxia than the WT mice. Similarly, using isolated perfused hearts, 5-HT4-TG mice hearts were more susceptible to ischemia compared to WT hearts. To study the influence of 5-HT4 receptors on cardiac hypertrophy, 5-HT4-TG mice were crossbred with TG mice overexpressing the catalytic subunit of PP2A in cardiac myocytes (PP2A-TG mice, a model for genetically induced hypertrophy). The cardiac contractility, determined by echocardiography, of the resulting double transgenic mice was attenuated like in the mono-transgenic PP2A-TG and, therefore, largely determined by the overexpression of PP2A. In summary, depending on the kind of stress put upon the animal or isolated tissue, 5-HT4 receptor overexpression could be either neutral (genetically induced hypertrophy, sepsis) or possibly detrimental (hypoxia, ischemia) for mechanical function. We suggest that depending on the underlying pathology, the activation or blockade of 5-HT4 receptors might offer novel drug therapy options in patients.
Keywords: 5-HT4 receptor; LPS; PP2A transgenic mice; cardiac hypertrophy; hypoxia; inflammation; ischemia; serotonin; transgenic mice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Cardiovascular effects of bufotenin on human 5-HT4 serotonin receptors in cardiac preparations of transgenic mice and in human atrial preparations.Naunyn Schmiedebergs Arch Pharmacol. 2023 Jul;396(7):1471-1485. doi: 10.1007/s00210-023-02414-8. Epub 2023 Feb 9. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 36754881 Free PMC article.
-
Cardiovascular effects of cisapride and prucalopride on human 5-HT4 receptors in transgenic mice.Naunyn Schmiedebergs Arch Pharmacol. 2018 Sep;391(9):975-985. doi: 10.1007/s00210-018-1519-z. Epub 2018 Jun 9. Naunyn Schmiedebergs Arch Pharmacol. 2018. PMID: 29947908
-
Cardiovascular effects of metoclopramide and domperidone on human 5-HT4-serotonin-receptors in transgenic mice and in human atrial preparations.Eur J Pharmacol. 2021 Jun 15;901:174074. doi: 10.1016/j.ejphar.2021.174074. Epub 2021 Mar 31. Eur J Pharmacol. 2021. PMID: 33811834
-
Characterization of Stressed Transgenic Mice Overexpressing H2-Histamine Receptors in the Heart.J Pharmacol Exp Ther. 2020 Sep;374(3):479-488. doi: 10.1124/jpet.120.000063. Epub 2020 Jun 19. J Pharmacol Exp Ther. 2020. PMID: 32561687
-
Desensitization of the human 5-HT4 receptor in isolated atria of transgenic mice.Naunyn Schmiedebergs Arch Pharmacol. 2017 Oct;390(10):987-996. doi: 10.1007/s00210-017-1403-2. Epub 2017 Jul 8. Naunyn Schmiedebergs Arch Pharmacol. 2017. PMID: 28689254
Cited by
-
Temperature alters the inotropic, chronotropic and proarrhythmic effects of histamine in atrial muscle preparations from humans and H2-receptor overexpressing mice.Naunyn Schmiedebergs Arch Pharmacol. 2023 Sep;396(9):2137-2150. doi: 10.1007/s00210-023-02457-x. Epub 2023 Mar 23. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 36951998 Free PMC article.
-
Cardiac Roles of Serotonin (5-HT) and 5-HT-Receptors in Health and Disease.Int J Mol Sci. 2023 Mar 1;24(5):4765. doi: 10.3390/ijms24054765. Int J Mol Sci. 2023. PMID: 36902195 Free PMC article. Review.
-
Cardiovascular effects of bufotenin on human 5-HT4 serotonin receptors in cardiac preparations of transgenic mice and in human atrial preparations.Naunyn Schmiedebergs Arch Pharmacol. 2023 Jul;396(7):1471-1485. doi: 10.1007/s00210-023-02414-8. Epub 2023 Feb 9. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 36754881 Free PMC article.
-
Correlation Analysis of Blood Glucose Level with Inflammatory Response and Immune Indicators in Patients with Sepsis.Dis Markers. 2022 May 26;2022:8779061. doi: 10.1155/2022/8779061. eCollection 2022. Dis Markers. 2022. PMID: 35664433 Free PMC article.
-
Protein Phosphatase 2A Improves Cardiac Functional Response to Ischemia and Sepsis.Int J Mol Sci. 2022 Apr 23;23(9):4688. doi: 10.3390/ijms23094688. Int J Mol Sci. 2022. PMID: 35563079 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

