Experimental Infection of the Biomphalaria glabrata Vector Snail by Schistosoma mansoni Parasites Drives Snail Microbiota Dysbiosis
- PMID: 34070104
- PMCID: PMC8158356
- DOI: 10.3390/microorganisms9051084
Experimental Infection of the Biomphalaria glabrata Vector Snail by Schistosoma mansoni Parasites Drives Snail Microbiota Dysbiosis
Abstract
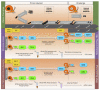
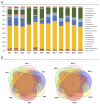
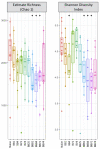
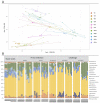
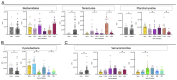
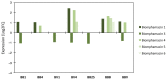
Host-parasite interaction can result in a strong alteration of the host-associated microbiota. This dysbiosis can affect the fitness of the host; can modify pathogen interaction and the outcome of diseases. Biomphalaria glabrata is the snail intermediate host of the trematode Schistosoma mansoni, the agent of human schistosomiasis, causing hundreds of thousands of deaths every year. Here, we present the first study of the snail bacterial microbiota in response to Schistosoma infection. We examined the interplay between B. glabrata, S. mansoni and host microbiota. Snails were infected and the microbiota composition was analysed by 16S rDNA amplicon sequencing approach. We demonstrated that the microbial composition of water did not affect the microbiota composition. Then, we characterised the Biomphalaria bacterial microbiota at the individual scale in both naive and infected snails. Sympatric and allopatric strains of parasites were used for infections and re-infections to analyse the modification or dysbiosis of snail microbiota in different host-parasite co-evolutionary contexts. Concomitantly, using RNAseq, we investigated the link between bacterial microbiota dysbiosis and snail anti-microbial peptide immune response. This work paves the way for a better understanding of snail/schistosome interaction and should have critical consequences in terms of snail control strategies for fighting schistosomiasis disease in the field.
Keywords: Biomphalaria snail; Schistosoma infection; bacteria; dysbiosis; immune response; microbiota.
Conflict of interest statement
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Figures
Similar articles
-
Compatibility Polymorphism Based on Long-Term Host-Parasite Relationships: Cross Talking Between Biomphalaria glabrata and the Trematode Schistosoma mansoni From Endemic Areas in Brazil.Front Immunol. 2019 Apr 9;10:328. doi: 10.3389/fimmu.2019.00328. eCollection 2019. Front Immunol. 2019. PMID: 31024517 Free PMC article.
-
Analysis of rhodopsin G protein-coupled receptor orthologs reveals semiochemical peptides for parasite (Schistosoma mansoni) and host (Biomphalaria glabrata) interplay.Sci Rep. 2022 May 17;12(1):8243. doi: 10.1038/s41598-022-11996-x. Sci Rep. 2022. PMID: 35581232 Free PMC article.
-
Immuno-molecular profile for Biomphalaria glabrata/Schistosoma mansoni interaction.Dev Comp Immunol. 2024 Jan;150:105083. doi: 10.1016/j.dci.2023.105083. Epub 2023 Oct 17. Dev Comp Immunol. 2024. PMID: 37852455 Review.
-
A Novel Toll-Like Receptor (TLR) Influences Compatibility between the Gastropod Biomphalaria glabrata, and the Digenean Trematode Schistosoma mansoni.PLoS Pathog. 2016 Mar 25;12(3):e1005513. doi: 10.1371/journal.ppat.1005513. eCollection 2016 Mar. PLoS Pathog. 2016. PMID: 27015424 Free PMC article.
-
Epigenetic modulation, stress and plasticity in susceptibility of the snail host, Biomphalaria glabrata, to Schistosoma mansoni infection.Int J Parasitol. 2016 Jun;46(7):389-94. doi: 10.1016/j.ijpara.2016.03.003. Epub 2016 Apr 4. Int J Parasitol. 2016. PMID: 27056272 Review.
Cited by
-
Assessing the microbiota of the snail intermediate host of trematodes, Galba truncatula.Parasit Vectors. 2024 Jan 23;17(1):31. doi: 10.1186/s13071-024-06118-7. Parasit Vectors. 2024. PMID: 38263069 Free PMC article.
-
Gut microbiota in parasite-transmitting gastropods.Infect Dis Poverty. 2023 Nov 24;12(1):105. doi: 10.1186/s40249-023-01159-z. Infect Dis Poverty. 2023. PMID: 38001502 Free PMC article. Review.
-
Organ-Specific Microbiomes of Biomphalaria Snails.bioRxiv [Preprint]. 2025 Jan 15:2024.06.11.598555. doi: 10.1101/2024.06.11.598555. bioRxiv. 2025. Update in: Anim Microbiome. 2025 Apr 24;7(1):40. doi: 10.1186/s42523-025-00403-1. PMID: 38915569 Free PMC article. Updated. Preprint.
-
Host-bacteriome transplants of the schistosome snail host Biomphalaria glabrata reflect species-specific associations.FEMS Microbiol Ecol. 2023 Aug 22;99(9):fiad101. doi: 10.1093/femsec/fiad101. FEMS Microbiol Ecol. 2023. PMID: 37632232 Free PMC article.
-
Metagenomic Analysis Reveals Variations in Gut Microbiomes of the Schistosoma mansoni-Transmitting Snails Biomphalaria straminea and Biomphalaria glabrata.Microorganisms. 2023 Sep 28;11(10):2419. doi: 10.3390/microorganisms11102419. Microorganisms. 2023. PMID: 37894077 Free PMC article.
References
-
- World Health Organization (WHO) TDR Strategic Direction for Research: Schistosomiasis. World Health Organization; Geneva, Switzerland: 2002.
-
- Doenhoff M.J., Hagan P., Cioli D., Southgate V., Pica-Mattoccia L., Botros S., Coles G., Tchuenté L.A.T., Mbaye A., Engels D. Praziquantel: Its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology. 2009;136:1825–1835. doi: 10.1017/S0031182009000493. - DOI - PubMed
-
- Williams G.M., Li Y.-S., Gray D.J., Zhao Z.-Y., Harn D.A., Shollenberger L.M., Li S.-M., Yu X., Feng Z., Guo J.-G., et al. Field Testing Integrated Interventions for Schistosomiasis Elimination in the People’s Republic of China: Outcomes of a Multifactorial Cluster-Randomized Controlled Trial. Front. Immunol. 2019;10:645. doi: 10.3389/fimmu.2019.00645. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

