The Role of ZEB2 in Human CD8 T Lymphocytes: Clinical and Cellular Immune Profiling in Mowat-Wilson Syndrome
- PMID: 34070208
- PMCID: PMC8158478
- DOI: 10.3390/ijms22105324
The Role of ZEB2 in Human CD8 T Lymphocytes: Clinical and Cellular Immune Profiling in Mowat-Wilson Syndrome
Abstract
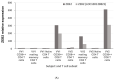
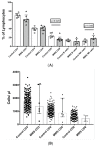
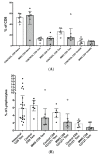
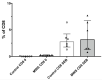
The Zeb2 gene encodes a transcription factor (ZEB2) that acts as an important immune mediator in mice, where it is expressed in early-activated effector CD8 T cells, and limits effector differentiation. Zeb2 homozygous knockout mice have deficits in CD8 T cells and NK cells. Mowat-Wilson syndrome (MWS) is a rare genetic disease resulting from heterozygous mutations in ZEB2 causing disease by haploinsufficiency. Whether ZEB2 exhibits similar expression patterns in human CD8 T cells is unknown, and MWS patients have not been comprehensively studied to identify changes in CD8 lymphocytes and NK cells, or manifestations of immunodeficiency. By using transcriptomic assessment, we demonstrated that ZEB2 is expressed in early-activated effector CD8 T cells of healthy human volunteers following vaccinia inoculation and found evidence of a role for TGFß-1/SMAD signaling in these cells. A broad immunological assessment of six genetically diagnosed MWS patients identified two patients with a history of recurrent sinopulmonary infections, one of whom had recurrent oral candidiasis, one with lymphopenia, two with thrombocytopenia and three with detectable anti-nuclear antibodies. Immunoglobulin levels, including functional antibody responses to protein and polysaccharide vaccination, were normal. The MWS patients had a significantly lower CD8 T cell subset as % of lymphocytes, compared to healthy controls (median 16.4% vs. 25%, p = 0.0048), and resulting increased CD4:CD8 ratio (2.6 vs. 1.8; p = 0.038). CD8 T cells responded normally to mitogen stimulation in vitro and memory CD8 T cells exhibited normal proportions of subsets with important tissue-specific homing markers and cytotoxic effector molecules. There was a trend towards a decrease in the CD8 T effector memory subset (3.3% vs. 5.9%; p = 0.19). NK cell subsets were normal. This is the first evidence that ZEB2 is expressed in early-activated human effector CD8 T cells, and that haploinsufficiency of ZEB2 in MWS patients had a slight effect on immune function, skewing T cells away from CD8 differentiation. To date there is insufficient evidence to support an immunodeficiency occurring in MWS patients.
Keywords: CD8 T cells; Mowat–Wilson syndrome; zinc finger E-box binding homeobox 2 gene (ZEB2).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Novel Zeb2 gene variation in the Mowat Wilson syndrome (MWS).J Pediatr Surg. 2016 Feb;51(2):268-71. doi: 10.1016/j.jpedsurg.2015.10.070. Epub 2015 Nov 5. J Pediatr Surg. 2016. PMID: 26852091
-
The spectrum of ZEB2 mutations causing the Mowat-Wilson syndrome in Japanese populations.Am J Med Genet A. 2014 Aug;164A(8):1899-908. doi: 10.1002/ajmg.a.36551. Epub 2014 Apr 8. Am J Med Genet A. 2014. PMID: 24715670
-
Neuropathology of Mowat-Wilson Syndrome.Pediatr Dev Pathol. 2020 Aug;23(4):322-325. doi: 10.1177/1093526620903956. Epub 2020 Apr 6. Pediatr Dev Pathol. 2020. PMID: 32252596
-
ZEB2, the Mowat-Wilson Syndrome Transcription Factor: Confirmations, Novel Functions, and Continuing Surprises.Genes (Basel). 2021 Jul 3;12(7):1037. doi: 10.3390/genes12071037. Genes (Basel). 2021. PMID: 34356053 Free PMC article. Review.
-
Mowat-Wilson Syndrome: Case Report and Review of ZEB2 Gene Variant Types, Protein Defects and Molecular Interactions.Int J Mol Sci. 2024 Feb 29;25(5):2838. doi: 10.3390/ijms25052838. Int J Mol Sci. 2024. PMID: 38474085 Free PMC article. Review.
Cited by
-
Multiomic profiling reveals metabolic alterations mediating aberrant platelet activity and inflammation in myeloproliferative neoplasms.J Clin Invest. 2024 Feb 1;134(3):e172256. doi: 10.1172/JCI172256. J Clin Invest. 2024. PMID: 38060311 Free PMC article.
-
Single-cell transcriptomics of melanoma sentinel lymph nodes identifies immune cell signatures associated with metastasis.JCI Insight. 2025 Mar 6;10(7):e183080. doi: 10.1172/jci.insight.183080. JCI Insight. 2025. PMID: 40048259 Free PMC article.
References
-
- Mowat D.R., Croaker G.D., Cass D.T., Kerr B.A., Chaitow J., Ades L.C., Chia N.L., Wilson M.J. Hirschsprung disease, microcephaly, mental retardation, and characteristic facial features: Delineation of a new syndrome and identification of a locus at chromosome 2q22-q23. J. Med. Genet. 1998;35:617–623. doi: 10.1136/jmg.35.8.617. - DOI - PMC - PubMed
-
- Ivanovski I., Djuric O., Caraffi S.G., Santodirocco D., Pollazzon M., Rosato S., Cordelli D.M., Abdalla E., Accorsi P., Adam M.P., et al. Phenotype and genotype of 87 patients with Mowat–Wilson syndrome and recommendations for care. Genet. Med. 2018;20:965–975. doi: 10.1038/gim.2017.221. - DOI - PubMed
-
- Omilusik K.D., Best J.A., Yu B., Goossens S., Weidemann A., Nguyen J.V., Seuntjens E., Stryjewska A., Zweier C., Roychoudhuri R., et al. Transcriptional repressor ZEB2 promotes terminal differentiation of CD8+ effector and memory T cell populations during infection. J. Exp. Med. 2015;212:2027–2039. doi: 10.1084/jem.20150194. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

