A Retrospective Analysis of Dabrafenib and/or Dabrafenib Plus Trametinib Combination in Patients with Metastatic Melanoma to Characterize Patients with Long-Term Benefit in the Individual Patient Program (DESCRIBE III)
- PMID: 34070224
- PMCID: PMC8158680
- DOI: 10.3390/cancers13102466
A Retrospective Analysis of Dabrafenib and/or Dabrafenib Plus Trametinib Combination in Patients with Metastatic Melanoma to Characterize Patients with Long-Term Benefit in the Individual Patient Program (DESCRIBE III)
Abstract
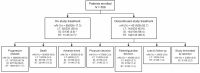
The dabrafenib plus trametinib (dab + tram) combination has demonstrated durable long-term efficacy in patients with BRAF V600-mutant metastatic melanoma. However, real-world data characterizing patients with long-term benefit are limited. DESCRIBE III was a global, observational, retrospective, chart review study in patients with unresectable or metastatic melanoma treated with dab monotherapy and/or dab + tram combination therapy as part of the Named Patient Program or Individual Patient Program. Overall, 509 patients were enrolled. Patients were categorized into three groups based on their observed treatment duration: long-term (on therapy ≥12 months), intermediate (on therapy ≥6 months and <12 months), and short-term (on therapy <6 months) duration of benefit. More patients in the short-term duration of benefit group had baseline characteristics associated with poor prognosis compared with the other two groups. Median lactate dehydrogenase (LDH) levels (368 U/L) at baseline were also higher in the short-term duration of benefit group. No new safety signals were identified. DESCRIBE III identified baseline characteristics associated with long-term benefit of dab + tram. Lower LDH level and <3 metastatic sites at baseline were associated with a longer duration of benefit, confirming that the findings from COMBI-d and COMBI-v are relevant to patients treated in a real-world setting.
Keywords: BRAF V600; chart review; dabrafenib; melanoma; real-world; trametinib.
Conflict of interest statement
V.G.A. received personal fees for advisory board and speaker fees from Bristol Myers Squibb (BMS), Merck, Merck Sharp & Dohme (MSD), Novartis, Pierre Fabre, and Roche and travel support from BMS, Novartis, and OncoSec outside the submitted work. P.Q. reports participation in advisory boards of BMS, MSD, Novartis, and Pierre Fabre. M.D.V. reports participation in advisory board and consultancy for BMS, Merck (MSD), Novartis, Pierre Fabre, and Sanofi. R.D. received personal fees from BMS, Merck, MSD, and Novartis outside the submitted work. F.C. reports personal fees for advisory board and speaker fees from BMS, Merck (MSD), and Novartis outside the submitted work. D.B. reports consulting and advisory role with BMS, MSD, Novartis, Roche-Genentech, and Pierre Fabre. P.F.F. received personal fees from BMS, MSD, Novartis, Pierre Fabre, and Roche outside the submitted work. S.T. reports lecture and conference registration fees sponsored by Eli Lilly, Johnson & Johnson, Merck (MSD), Novartis, Roche, and Servier. I.K. received personal fees for advisory boards from BMS, MSD, and Sanofi outside the submitted work. P.A.A. received grant/research funds from Array, BMS, Roche-Genentech, and Sanofi; personal fees for consultant/advisory role from Alkermes, Array, AstraZeneca, BMS, Boehringer Ingelheim, Daiichi Sankyo, Eisai, Idera, Immunocore, Incyte, Italfarmaco, Lunaphore, MedImmune, Merck Serono, MSD, Nektar, Nouscom, Novartis, Oncosec, Pfizer, Pierre Fabre, Regeneron, Roche-Genentech, Sandoz, Sanofi, Sun Pharma, Syndax, Takis, Ultimovacs, and 4SC; and travel support from MSD outside the submitted work. A.A. received personal fees for consultancy, advisory board, or speaker fees from Amgen, BMS, Merck, MSD, Novartis, Pierre Fabre, Roche, and Sanofi outside the submitted work. H.G. received grant and personal fees as honoraria and advisory role from Amgen, BMS, MSD, Novartis, and Pierre Fabre and travel support from BMS, MSD, Pierre Fabre, and Roche outside the submitted work. H.B. is an employee of Novartis. T.S. is an employee and shareholder of Novartis. E.d.J. is an employee of Novartis. B.N. reports grant to his institution from Novartis and personal fees for participation in advisory board meetings from BMS, MSD, Novartis, and Pfizer outside the submitted work. M.A. and R.G. have declared no conflicts of interest.
Figures
References
-
- Robert C., Grob J.J., Stroyakovskiy D., Karaszewska B., Hauschild A., Levchenko E., Chiarion Sileni V., Schachter J., Garbe C., Bondarenko I., et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N. Engl. J. Med. 2019;381:626–636. doi: 10.1056/NEJMoa1904059. - DOI - PubMed
-
- Martin-Algarra S., Hinshelwood R., Mesnage S., Cebon J., Ferrucci P.F., Aglietta M., Neyns B., Chiarion-Sileni V., Lindsay C.R., Del Vecchio M., et al. Effectiveness of dabrafenib in the treatment of patients with BRAF V600-mutated metastatic melanoma in a named patient program. Melanoma Res. 2019;29:527–532. doi: 10.1097/CMR.0000000000000608. - DOI - PubMed
-
- Atkinson V., Sandhu S., Hospers G., Long G.V., Aglietta M., Ferrucci P.F., Tulyte S., Cappellini G.C.A., Soriano V., Ali S., et al. Dabrafenib plus trametinib is effective in the treatment of BRAF V600-mutated metastatic melanoma patients: Analysis of patients from the dabrafenib plus trametinib named patient program (DESCRIBE II) Melanoma Res. 2020;30:261–267. doi: 10.1097/CMR.0000000000000654. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

