Generation of Therapeutically Potent Spheroids from Human Endometrial Mesenchymal Stem/Stromal Cells
- PMID: 34070346
- PMCID: PMC8229788
- DOI: 10.3390/jpm11060466
Generation of Therapeutically Potent Spheroids from Human Endometrial Mesenchymal Stem/Stromal Cells
Abstract
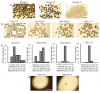
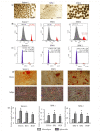
Endometrial mesenchymal stem/stromal cells (eMSCs) hold great promise in bioengineering and regenerative medicine due to their high expansion potential, unique immunosuppressive properties and multilineage differentiation capacity. Usually, eMSCs are maintained and applied as a monolayer culture. Recently, using animal models with endometrial and skin defects, we showed that formation of multicellular aggregates known as spheroids from eMSCs enhances their tissue repair capabilities. In this work, we refined a method of spheroid formation, which makes it possible to obtain well-formed aggregates with a narrow size distribution both at early eMSC passages and after prolonged cultivation. The use of serum-free media allows this method to be used for the production of spheroids for clinical purposes. Wound healing experiments on animals confirmed the high therapeutic potency of the produced eMSC spheroids in comparison to the monolayer eMSC culture.
Keywords: cell spheroids; endometrial mesenchymal stem cells; serum-free conditions; wound healing.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Three-Dimensional Compaction Switches Stress Response Programs and Enhances Therapeutic Efficacy of Endometrial Mesenchymal Stem/Stromal Cells.Front Cell Dev Biol. 2020 Jun 16;8:473. doi: 10.3389/fcell.2020.00473. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32612993 Free PMC article.
-
Paracrine and Autocrine Effects of VEGF Are Enhanced in Human eMSC Spheroids.Int J Mol Sci. 2022 Nov 18;23(22):14324. doi: 10.3390/ijms232214324. Int J Mol Sci. 2022. PMID: 36430800 Free PMC article.
-
Human mesenchymal stem cells in spheroids improve fertility in model animals with damaged endometrium.Stem Cell Res Ther. 2018 Feb 26;9(1):50. doi: 10.1186/s13287-018-0801-9. Stem Cell Res Ther. 2018. PMID: 29482664 Free PMC article.
-
Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application.Front Cell Dev Biol. 2020 Jul 9;8:497. doi: 10.3389/fcell.2020.00497. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32742977 Free PMC article. Review.
-
Endometrial stem/progenitor cells: the first 10 years.Hum Reprod Update. 2016 Mar-Apr;22(2):137-63. doi: 10.1093/humupd/dmv051. Epub 2015 Nov 9. Hum Reprod Update. 2016. PMID: 26552890 Free PMC article. Review.
Cited by
-
Endometrial stem/progenitor cells: Properties, origins, and functions.Genes Dis. 2022 Aug 31;10(3):931-947. doi: 10.1016/j.gendis.2022.08.009. eCollection 2023 May. Genes Dis. 2022. PMID: 37396532 Free PMC article. Review.
-
Formation of ovarian organoid by co-culture of human endometrial mesenchymal stem cells and mouse oocyte in 3-dimensional culture system.Cytotechnology. 2024 Oct;76(5):571-584. doi: 10.1007/s10616-024-00639-w. Epub 2024 Jun 24. Cytotechnology. 2024. PMID: 39188652
-
Mesenchymal Stem/Stromal Cells in Three-Dimensional Cell Culture: Ion Homeostasis and Ouabain-Induced Apoptosis.Biomedicines. 2023 Jan 21;11(2):301. doi: 10.3390/biomedicines11020301. Biomedicines. 2023. PMID: 36830836 Free PMC article.
-
Piezo1 Ion Channels Regulate the Formation and Spreading of Human Endometrial Mesenchymal Stem Cell Spheroids.Int J Mol Sci. 2025 Mar 10;26(6):2474. doi: 10.3390/ijms26062474. Int J Mol Sci. 2025. PMID: 40141118 Free PMC article.
-
Endometrial Stem/Progenitor Cells: Prospects and Challenges.J Pers Med. 2022 Sep 7;12(9):1466. doi: 10.3390/jpm12091466. J Pers Med. 2022. PMID: 36143251 Free PMC article.
References
-
- Dominici M., Blanc K.L., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. - DOI - PubMed
LinkOut - more resources
Full Text Sources

