Complex Interplay of Genes Underlies Invasiveness in Fibrosarcoma Progression Model
- PMID: 34070472
- PMCID: PMC8197499
- DOI: 10.3390/jcm10112297
Complex Interplay of Genes Underlies Invasiveness in Fibrosarcoma Progression Model
Abstract
Sarcomas are a heterogeneous group of mesenchymal tumours, with a great variability in their clinical behaviour. While our knowledge of sarcoma initiation has advanced rapidly in recent years, relatively little is known about mechanisms of sarcoma progression. JUN-murine fibrosarcoma progression series consists of four sarcoma cell lines, JUN-1, JUN-2, JUN-2fos-3, and JUN-3. JUN-1 and -2 were established from a single tumour initiated in a H2K/v-jun transgenic mouse, JUN-3 originates from a different tumour in the same animal, and JUN-2fos-3 results from a targeted in vitro transformation of the JUN-2 cell line. The JUN-1, -2, and -3 cell lines represent a linear progression from the least transformed JUN-2 to the most transformed JUN-3, with regard to all the transformation characteristics studied, while the JUN-2fos-3 cell line exhibits a unique transformation mode, with little deregulation of cell growth and proliferation, but pronounced motility and invasiveness. The invasive sarcoma sublines JUN-2fos-3 and JUN-3 show complex metabolic profiles, with activation of both mitochondrial oxidative phosphorylation and glycolysis and a significant increase in spared respiratory capacity. The specific transcriptomic profile of invasive sublines features very complex biological relationships across the identified genes and proteins, with accentuated autocrine control of motility and angiogenesis. Pharmacologic inhibition of one of the autocrine motility factors identified, Ccl8, significantly diminished both motility and invasiveness of the highly transformed fibrosarcoma cell. This progression series could be greatly valuable for deciphering crucial aspects of sarcoma progression and defining new prognostic markers and potential therapeutic targets.
Keywords: Ccl8; fibrosarcoma; invasiveness; progression series; transcriptome.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
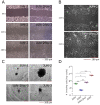

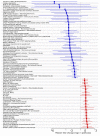

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

