Therapeutic Targets and Tumor Microenvironment in Colorectal Cancer
- PMID: 34070480
- PMCID: PMC8197564
- DOI: 10.3390/jcm10112295
Therapeutic Targets and Tumor Microenvironment in Colorectal Cancer
Abstract
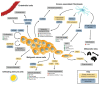
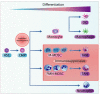
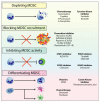
Colorectal cancer (CRC) is a genetically, anatomically, and transcriptionally heterogeneous disease. The prognosis for a CRC patient depends on the stage of the tumor at diagnosis and widely differs accordingly. The tumor microenvironment (TME) in CRC is an important factor affecting targeted cancer therapy. The TME has a dynamic composition including various cell types, such as cancer-associated fibroblasts, tumor-associated macrophages, regulatory T cells, and myeloid-derived suppressor cells, as well as extracellular factors that surround cancer cells and have functional and structural roles under physiological and pathological conditions. Moreover, the TME can limit the efficacy of therapeutic agents through high interstitial pressure, fibrosis, and the degradation of the therapeutic agents by enzymatic activity. For this reason, the TME is a fertile ground for the discovery of new drugs. The aim of this narrative review is to present current knowledge and future perspectives regarding the TME composition based on strategies for patients with CRC.
Keywords: angiogenesis; cancer-associated fibroblasts; colorectal cancer; regulatory T cells; targeted therapy; tumor microenvironment; tumor-associated macrophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Gallo G., Sena G., Vescio G., Papandrea M., Sacco R., Trompetto M., Sammarco G. The prognostic value of KRAS and BRAF in stage I-III colorectal cancer. A systematic review. Ann. Ital. Chir. 2019;90:127–137. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

