Protein Engineering of Electron Transfer Components from Electroactive Geobacter Bacteria
- PMID: 34070486
- PMCID: PMC8227773
- DOI: 10.3390/antiox10060844
Protein Engineering of Electron Transfer Components from Electroactive Geobacter Bacteria
Abstract
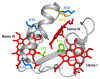
Electrogenic microorganisms possess unique redox biological features, being capable of transferring electrons to the cell exterior and converting highly toxic compounds into nonhazardous forms. These microorganisms have led to the development of Microbial Electrochemical Technologies (METs), which include applications in the fields of bioremediation and bioenergy production. The optimization of these technologies involves efforts from several different disciplines, ranging from microbiology to materials science. Geobacter bacteria have served as a model for understanding the mechanisms underlying the phenomenon of extracellular electron transfer, which is highly dependent on a multitude of multiheme cytochromes (MCs). MCs are, therefore, logical targets for rational protein engineering to improve the extracellular electron transfer rates of these bacteria. However, the presence of several heme groups complicates the detailed redox characterization of MCs. In this Review, the main characteristics of electroactive Geobacter bacteria, their potential to develop microbial electrochemical technologies and the main features of MCs are initially highlighted. This is followed by a detailed description of the current methodologies that assist the characterization of the functional redox networks in MCs. Finally, it is discussed how this information can be explored to design optimal Geobacter-mutated strains with improved capabilities in METs.
Keywords: Microbial Electrochemical Technologies (METs); Nuclear Magnetic Resonance (NMR); bioenergy production; bioremediation; electroactive microorganisms; multiheme cytochromes; protein engineering; redox characterization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

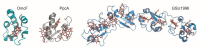
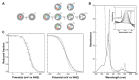
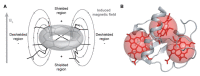
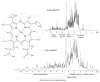





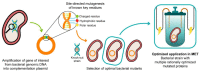
References
-
- Potter M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. Lond. Ser. B Contain. Pap. Biol. Character. 1911;84:260–276.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

