Nature of Linear Spectral Properties and Fast Electronic Relaxations in Green Fluorescent Pyrrolo[3,4-c]Pyridine Derivative
- PMID: 34070488
- PMCID: PMC8197551
- DOI: 10.3390/ijms22115592
Nature of Linear Spectral Properties and Fast Electronic Relaxations in Green Fluorescent Pyrrolo[3,4-c]Pyridine Derivative
Abstract
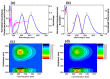
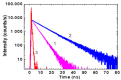
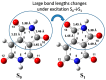
The electronic nature of 4-hydroxy-1H-pyrrolo[3,4-c]pyridine-1,3,6(2H,5H)-trione (HPPT) was comprehensively investigated in liquid media at room temperature using steady-state and time-resolved femtosecond transient absorption spectroscopic techniques. The analysis of the linear photophysical and photochemical parameters of HPPT, including steady-state absorption, fluorescence and excitation anisotropy spectra, along with the lifetimes of fluorescence emission and photodecomposition quantum yields, revealed the nature of its large Stokes shift, specific changes in the permanent dipole moments under electronic excitation, weak dipole transitions with partially anisotropic character, and high photostability. Transient absorption spectra of HPPT were obtained with femtosecond resolution and no characteristic solvate relaxation processes in protic (methanol) solvent were revealed. Efficient light amplification (gain) was observed in the fluorescence spectral range of HPPT, but no super-luminescence and lasing phenomena were detected. The electronic structure of HPPT was also analyzed with quantum-chemical calculations using a DFT/B3LYP method and good agreement with experimental data was shown. The development and investigation of new pyrrolo[3,4-c]pyridine derivatives are important due to their promising fluorescent properties and potential for use in physiological applications.
Keywords: femtosecond transient absorption spectroscopy; linear spectral properties; pyrrolo[3,4-c]pyridine derivative; quantum chemical analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
New Two-Photon Absorbing Squaraine Derivative with Efficient Near-Infrared Fluorescence, Superluminescence, and High Photostability.J Phys Chem B. 2022 Jun 2;126(21):3897-3907. doi: 10.1021/acs.jpcb.2c01288. Epub 2022 May 18. J Phys Chem B. 2022. PMID: 35584210
-
Superfluorescent squaraine with efficient two-photon absorption and high photostability.Chemphyschem. 2013 Oct 21;14(15):3532-42. doi: 10.1002/cphc.201300447. Epub 2013 Sep 10. Chemphyschem. 2013. PMID: 24022985
-
Nature of Linear Spectral Properties and Fast Relaxations in the Excited States and Two-Photon Absorption Efficiency of 3-Thiazolyl and 3-Phenyltiazolyl Coumarin Derivatives.ACS Omega. 2023 Mar 16;8(12):11564-11573. doi: 10.1021/acsomega.3c00654. eCollection 2023 Mar 28. ACS Omega. 2023. PMID: 37008079 Free PMC article.
-
Design and electronic structure of new styryl dye bases: steady-state and time-resolved spectroscopic studies.J Phys Chem A. 2014 Jun 26;118(25):4502-9. doi: 10.1021/jp503263f. Epub 2014 Jun 11. J Phys Chem A. 2014. PMID: 24918283
-
Naphthalene and its Derivatives: Efficient Fluorescence Probes for Detecting and Imaging Purposes.J Fluoresc. 2023 Jul;33(4):1273-1303. doi: 10.1007/s10895-023-03153-y. Epub 2023 Feb 3. J Fluoresc. 2023. PMID: 36735102 Review.
Cited by
-
Exploring the Impact of Nitrogen Doping on the Optical Properties of Carbon Dots Synthesized from Citric Acid.Nanomaterials (Basel). 2023 Apr 12;13(8):1344. doi: 10.3390/nano13081344. Nanomaterials (Basel). 2023. PMID: 37110929 Free PMC article.
-
Synthesis of Pyridin-1(2H)-ylacrylates and the Effects of Different Functional Groups on Their Fluorescence.Molecules. 2023 Sep 8;28(18):6511. doi: 10.3390/molecules28186511. Molecules. 2023. PMID: 37764287 Free PMC article.
References
-
- Liao J., Xu Y., Zhao H., Zong Q., Fang Y. Novel A-D-A type small molecules with b-alkynylated BODIPY flanks for bulk heterojunction solar cells. Org. Electron. 2017;49:321–333. doi: 10.1016/j.orgel.2017.06.054. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

