Serum Amyloid A1/Toll-Like Receptor-4 Axis, an Important Link between Inflammation and Outcome of TBI Patients
- PMID: 34070533
- PMCID: PMC8227125
- DOI: 10.3390/biomedicines9060599
Serum Amyloid A1/Toll-Like Receptor-4 Axis, an Important Link between Inflammation and Outcome of TBI Patients
Abstract
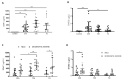
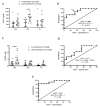
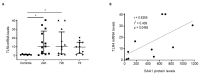
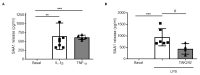
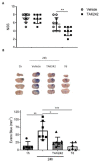
Traumatic brain injury (TBI) is one of the leading causes of mortality and disability worldwide without any validated biomarker or set of biomarkers to help the diagnosis and evaluation of the evolution/prognosis of TBI patients. To achieve this aim, a deeper knowledge of the biochemical and pathophysiological processes triggered after the trauma is essential. Here, we identified the serum amyloid A1 protein-Toll-like receptor 4 (SAA1-TLR4) axis as an important link between inflammation and the outcome of TBI patients. Using serum and mRNA from white blood cells (WBC) of TBI patients, we found a positive correlation between serum SAA1 levels and injury severity, as well as with the 6-month outcome of TBI patients. SAA1 levels also correlate with the presence of TLR4 mRNA in WBC. In vitro, we found that SAA1 contributes to inflammation via TLR4 activation that releases inflammatory cytokines, which in turn increases SAA1 levels, establishing a positive proinflammatory loop. In vivo, post-TBI treatment with the TLR4-antagonist TAK242 reduces SAA1 levels, improves neurobehavioral outcome, and prevents blood-brain barrier disruption. Our data support further evaluation of (i) post-TBI treatment in the presence of TLR4 inhibition for limiting TBI-induced damage and (ii) SAA1-TLR4 as a biomarker of injury progression in TBI patients.
Keywords: biomarkers; immunomodulation; neuroinflammation; prognosis; traumatic brain injury.
Conflict of interest statement
Some results of the study have been included in a pending patent.
Figures


References
-
- Jarrahi A., Braun M., Ahluwalia M., Gupta R.V., Wilson M., Munie S., Ahluwalia P., Vender J.R., Vale F.L., Dhandapani K.M., et al. Revisiting traumatic brain injury: From molecular mechanisms to therapeutic interventions. Biomedicines. 2020;8:389. doi: 10.3390/biomedicines8100389. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

