The Effect of Inflammatory Priming on the Therapeutic Potential of Mesenchymal Stromal Cells for Spinal Cord Repair
- PMID: 34070547
- PMCID: PMC8227154
- DOI: 10.3390/cells10061316
The Effect of Inflammatory Priming on the Therapeutic Potential of Mesenchymal Stromal Cells for Spinal Cord Repair
Abstract
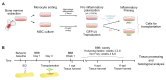
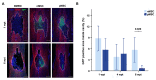
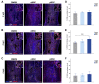
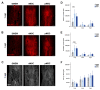
Mesenchymal stromal cells (MSC) are used for cell therapy for spinal cord injury (SCI) because of their ability to support tissue repair by paracrine signaling. Preclinical and clinical research testing MSC transplants for SCI have revealed limited success, which warrants the exploration of strategies to improve their therapeutic efficacy. MSC are sensitive to the microenvironment and their secretome can be altered in vitro by exposure to different culture media. Priming MSC with inflammatory stimuli increases the expression and secretion of reparative molecules. We studied the effect of macrophage-derived inflammation priming on MSC transplants and of primed MSC (pMSC) acute transplants (3 days) on spinal cord repair using an adult rat model of moderate-severe contusive SCI. We found a decrease in long-term survival of pMSC transplants compared with unprimed MSC transplants. With a pMSC transplant, we found significantly more anti-inflammatory macrophages in the contusion at 4 weeks post transplantation (wpt). Blood vessel presence and maturation in the contusion at 1 wpt was similar in rats that received pMSC or untreated MSC. Nervous tissue sparing and functional recovery were similar across groups. Our results indicate that macrophage-derived inflammation priming does not increase the overall therapeutic potential of an MSC transplant in the adult rat contused spinal cord.
Keywords: MSC; SCI; angiogenesis; cell therapy; contusion; immunomodulation; macrophages; neuroprotection; repair.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Johnson T.V., DeKorver N.W., Levasseur V.A., Osborne A., Tassoni A., Lorber B., Heller J.P., Villasmil R., Bull N.D., Martin K.R., et al. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain. 2014;137:503–519. doi: 10.1093/brain/awt292. - DOI - PMC - PubMed
-
- Asami T., Ishii M., Fujii H., Namkoong H., Tasaka S., Matsushita K., Ishii K., Yagi K., Fujiwara H., Funatsu Y., et al. Modulation of murine macrophage TLR7/8-mediated cytokine expression by mesenchymal stem cell-conditioned medium. Mediat. Inflamm. 2013;2013:264260. doi: 10.1155/2013/264260. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

