Synthesis and Biological Evaluation of Spirocyclic Chromane Derivatives as a Potential Treatment of Prostate Cancer
- PMID: 34070610
- PMCID: PMC8198214
- DOI: 10.3390/molecules26113162
Synthesis and Biological Evaluation of Spirocyclic Chromane Derivatives as a Potential Treatment of Prostate Cancer
Abstract
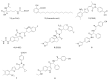
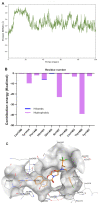
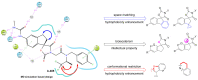
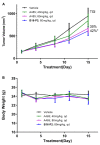
As a significant co-activator involved in cell cycle and cell growth, differentiation and development, p300/CBP has shown extraordinary potential target in cancer therapy. Herein we designed new compounds from the lead compound A-485 based on molecular dynamic simulations. A series of new spirocyclic chroman derivatives was prepared, characterized and proven to be a potential treatment of prostate cancer. The most potent compound B16 inhibited the proliferation of enzalutamide-resistant 22Rv1 cells with an IC50 value of 96 nM. Furthermore, compounds B16-P2 displayed favorable overall pharmacokinetic profiles, and better tumor growth inhibition than A-485 in an in vivo xenograft model.
Keywords: HAT inhibitors; antitumor activity; p300/CBP.
Conflict of interest statement
The author declares no conflict of interest.
Figures

Similar articles
-
Design, Synthesis, and Biological Evaluation of 1-(Indolizin-3-yl)ethan-1-ones as CBP Bromodomain Inhibitors for the Treatment of Prostate Cancer.J Med Chem. 2022 Jan 13;65(1):785-810. doi: 10.1021/acs.jmedchem.1c01864. Epub 2021 Dec 28. J Med Chem. 2022. PMID: 34962793
-
1-Piperazinylphthalazines as potential VEGFR-2 inhibitors and anticancer agents: Synthesis and in vitro biological evaluation.Eur J Med Chem. 2016 Jan 1;107:165-79. doi: 10.1016/j.ejmech.2015.10.053. Epub 2015 Nov 5. Eur J Med Chem. 2016. PMID: 26590508
-
Discovery and optimization of 1-(1H-indol-1-yl)ethanone derivatives as CBP/EP300 bromodomain inhibitors for the treatment of castration-resistant prostate cancer.Eur J Med Chem. 2018 Mar 10;147:238-252. doi: 10.1016/j.ejmech.2018.01.087. Epub 2018 Feb 6. Eur J Med Chem. 2018. PMID: 29448139
-
Discovery of Highly Potent, Selective, and Orally Efficacious p300/CBP Histone Acetyltransferases Inhibitors.J Med Chem. 2020 Feb 13;63(3):1337-1360. doi: 10.1021/acs.jmedchem.9b01721. Epub 2020 Jan 28. J Med Chem. 2020. PMID: 31910017
-
Design, synthesis and biological evaluation of novel chroman derivatives as non-selective acetyl-CoA carboxylase inhibitors.Bioorg Chem. 2020 Aug;101:103943. doi: 10.1016/j.bioorg.2020.103943. Epub 2020 May 15. Bioorg Chem. 2020. PMID: 32554277
Cited by
-
The role of histone post-translational modifications in cancer and cancer immunity: functions, mechanisms and therapeutic implications.Front Immunol. 2024 Nov 15;15:1495221. doi: 10.3389/fimmu.2024.1495221. eCollection 2024. Front Immunol. 2024. PMID: 39620228 Free PMC article. Review.
-
KATs off: Biomedical insights from lysine acetyltransferase inhibitors.Curr Opin Chem Biol. 2023 Feb;72:102255. doi: 10.1016/j.cbpa.2022.102255. Epub 2022 Dec 28. Curr Opin Chem Biol. 2023. PMID: 36584580 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

