Garlic Essential Oil as Promising Option for the Treatment of Acute Campylobacteriosis-Results from a Preclinical Placebo-Controlled Intervention Study
- PMID: 34070612
- PMCID: PMC8227651
- DOI: 10.3390/microorganisms9061140
Garlic Essential Oil as Promising Option for the Treatment of Acute Campylobacteriosis-Results from a Preclinical Placebo-Controlled Intervention Study
Abstract
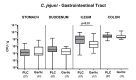
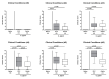
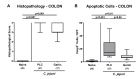
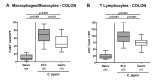
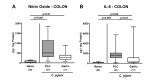
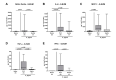
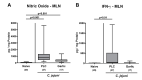
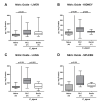
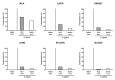
Since human infections with Campylobacter jejuni including antibiotic-resistant strains are rising worldwide, natural compounds might constitute promising antibiotics-independent treatment options for campylobacteriosis. Since the health-beneficial properties of garlic have been known for centuries, we here surveyed the antimicrobial and immune-modulatory effects of garlic essential oil (EO) in acute experimental campylobacteriosis. Therefore, secondary abiotic IL-10-/- mice were orally infected with C. jejuni strain 81-176 and garlic-EO treatment via the drinking water was initiated on day 2 post-infection. Mice from the garlic-EO group displayed less severe clinical signs of acute campylobacteriosis as compared to placebo counterparts that were associated with lower ileal C. jejuni burdens on day 6 post-infection. Furthermore, when compared to placebo application, garlic-EO treatment resulted in alleviated colonic epithelia cell apoptosis, in less pronounced C. jejuni induced immune cell responses in the large intestines, in dampened pro-inflammatory mediator secretion in intestinal and extra-intestinal compartments, and, finally, in less frequent translocation of viable pathogens from the intestines to distinct organs. Given its potent immune-modulatory and disease-alleviating effects as shown in our actual preclinical placebo-controlled intervention study, we conclude that garlic-EO may be considered as promising adjunct treatment option for acute campylobacteriosis in humans.
Keywords: Campylobacter jejuni; acute campylobacteriosis model; enteropathogenic infection; garlic essential oil; host-pathogen interaction; immune-modulatory effects; natural antibiotics-independent compounds; preclinical placebo-controlled intervention study; secondary abiotic IL-10-/- mice.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Backert S., Tegtmeyer N., Cróinín T.Ó., Boehm M., Heimesaat M.M. Human campylobacteriosis. In: Klein G., editor. Campylobacter. Academic Press; Cambridge, MA, USA: 2017. pp. 1–25. - DOI
-
- Mousavi S., Bereswill S., Heimesaat M.M. Novel Clinical Campylobacter jejuni Infection Models Based on Sensitization of Mice to Lipooligosaccharide, a Major Bacterial Factor Triggering Innate Immune Responses in Human Campylobacteriosis. Microorganisms. 2020;8:482. doi: 10.3390/microorganisms8040482. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

