Synthesis of Novel Halogenated Heterocycles Based on o-Phenylenediamine and Their Interactions with the Catalytic Subunit of Protein Kinase CK2
- PMID: 34070615
- PMCID: PMC8198750
- DOI: 10.3390/molecules26113163
Synthesis of Novel Halogenated Heterocycles Based on o-Phenylenediamine and Their Interactions with the Catalytic Subunit of Protein Kinase CK2
Abstract
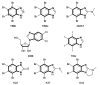
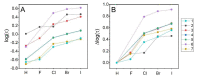
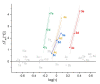
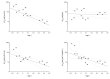
Protein kinase CK2 is a highly pleiotropic protein kinase capable of phosphorylating hundreds of protein substrates. It is involved in numerous cellular functions, including cell viability, apoptosis, cell proliferation and survival, angiogenesis, or ER-stress response. As CK2 activity is found perturbed in many pathological states, including cancers, it becomes an attractive target for the pharma. A large number of low-mass ATP-competitive inhibitors have already been developed, the majority of them halogenated. We tested the binding of six series of halogenated heterocyclic ligands derived from the commercially available 4,5-dihalo-benzene-1,2-diamines. These ligand series were selected to enable the separation of the scaffold effect from the hydrophobic interactions attributed directly to the presence of halogen atoms. In silico molecular docking was initially applied to test the capability of each ligand for binding at the ATP-binding site of CK2. HPLC-derived ligand hydrophobicity data are compared with the binding affinity assessed by low-volume differential scanning fluorimetry (nanoDSF). We identified three promising ligand scaffolds, two of which have not yet been described as CK2 inhibitors but may lead to potent CK2 kinase inhibitors. The inhibitory activity against CK2α and toxicity against four reference cell lines have been determined for eight compounds identified as the most promising in nanoDSF assay.
Keywords: cell toxicity; differential scanning fluorimetry; halogenated heterocycles; hydrophobic contribution; inhibitory activity; kinase CK2; ligand binding; molecular modeling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A competition between hydrophobic and electrostatic interactions in protein-ligand systems. Binding of heterogeneously halogenated benzotriazoles by the catalytic subunit of human protein kinase CK2.IUBMB Life. 2020 Jun;72(6):1211-1219. doi: 10.1002/iub.2271. Epub 2020 Mar 12. IUBMB Life. 2020. PMID: 32162783
-
Thermodynamics parameters for binding of halogenated benzotriazole inhibitors of human protein kinase CK2α.Biochim Biophys Acta. 2015 Oct;1854(10 Pt B):1708-17. doi: 10.1016/j.bbapap.2015.04.004. Epub 2015 Apr 17. Biochim Biophys Acta. 2015. PMID: 25891901
-
Halogen Atoms in the Protein-Ligand System. Structural and Thermodynamic Studies of the Binding of Bromobenzotriazoles by the Catalytic Subunit of Human Protein Kinase CK2.J Phys Chem B. 2021 Mar 18;125(10):2491-2503. doi: 10.1021/acs.jpcb.0c10264. Epub 2021 Mar 9. J Phys Chem B. 2021. PMID: 33689348 Free PMC article.
-
Protein kinase CK2 in health and disease: Structural bases of protein kinase CK2 inhibition.Cell Mol Life Sci. 2009 Jun;66(11-12):1868-89. doi: 10.1007/s00018-009-9155-x. Cell Mol Life Sci. 2009. PMID: 19387547 Free PMC article. Review.
-
Features and potentials of ATP-site directed CK2 inhibitors.Biochim Biophys Acta. 2005 Dec 30;1754(1-2):263-70. doi: 10.1016/j.bbapap.2005.07.043. Epub 2005 Sep 12. Biochim Biophys Acta. 2005. PMID: 16198160 Review.
Cited by
-
Differential scanning fluorimetry followed by microscale thermophoresis and/or isothermal titration calorimetry as an efficient tool for ligand screening.Biophys Rev. 2025 Feb 13;17(1):199-223. doi: 10.1007/s12551-025-01280-3. eCollection 2025 Feb. Biophys Rev. 2025. PMID: 40060009 Free PMC article. Review.
-
Regio-controllable [2+2] benzannulation with two adjacent C(sp3)-H bonds.Science. 2023 May 12;380(6645):639-644. doi: 10.1126/science.adg5282. Epub 2023 May 11. Science. 2023. PMID: 37167386 Free PMC article.
-
Recent Advances in the Discovery of CK2 Inhibitors.ACS Omega. 2024 May 3;9(19):20702-20719. doi: 10.1021/acsomega.3c10478. eCollection 2024 May 14. ACS Omega. 2024. PMID: 38764653 Free PMC article. Review.
-
5,6-diiodo-1H-benzotriazole: new TBBt analogue that minutely affects mitochondrial activity.Sci Rep. 2021 Dec 8;11(1):23701. doi: 10.1038/s41598-021-03136-8. Sci Rep. 2021. PMID: 34880390 Free PMC article.
References
-
- Krippner-Heidenreich A., Talanian R.V., Sekul R., Kraft R., Thole H., Ottleben H., Luscher B. Targeting of the transcription factor Max during apoptosis: Phosphorylation-regulated cleavage by caspase-5 at an unusual glutamic acid residue in position P1. Biochem. J. 2001;358:705–715. doi: 10.1042/bj3580705. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

