Synthesis of Functionalized Diethyl(pyrrolidin-2-yl)phosphonate and Diethyl(5-oxopyrrolidin-2-yl)phosphonate
- PMID: 34070623
- PMCID: PMC8197975
- DOI: 10.3390/molecules26113160
Synthesis of Functionalized Diethyl(pyrrolidin-2-yl)phosphonate and Diethyl(5-oxopyrrolidin-2-yl)phosphonate
Abstract
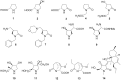
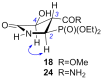
Short and efficient syntheses of functionalized (pyrrolidin-2-yl)phosphonate and (5-oxopyrrolidin-2-yl)phosphonate have been developed. The synthetic strategy involved the diastereospecific 1,3-dipolar cycloaddition of N-benzyl-C-(diethoxyphosphoryl)nitrone to cis-1,4-dihydroxybut-2-ene and dimethyl maleate, respectively. O,O-Diethyl 3-carbamoyl-4-hydroxy(5-oxopyrrolidin-2-yl)phosphonate was obtained from O,O-diethyl 2-benzyl-4,5-dimethoxycarbonyl(isoxazolidin-3-yl)phosphonate by hydrogenation and subsequent treatment with ammonia, whereas transformation of O,O-diethyl 2-benzyl-4,5-dihydroxymethyl(isoxazolidin-3-yl)phosphonate into O,O-diethyl 3-aminomethyl-4-hydroxy(pyrrolidin-2-yl)phosphonate was accomplished by mesylation followed by hydrogenolysis to undergo intramolecular cyclization and the introduction of amino group via ammonolysis. Stereochemistry of the isoxazolidine cycloadducts, as well as the final functionalized (pyrrolidin-2-yl)- and (5-oxopyrrolidin-2-yl)phosphonates were established based on conformational analyses using vicinal H-H, H-P, and C-P couplings and supported by the observed diagnostic NOESY correlation signals.
Keywords: cycloaddition; isoxazolidines; phosphonates; substituted pyrrolidines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
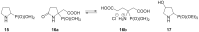
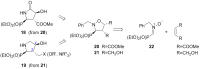
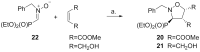
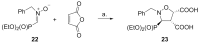
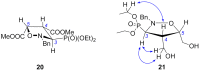
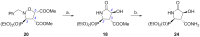
Similar articles
-
Synthesis of Diversely Substituted Diethyl (Pyrrolidin-2-Yl)Phosphonates.Molecules. 2025 May 7;30(9):2078. doi: 10.3390/molecules30092078. Molecules. 2025. PMID: 40363884 Free PMC article.
-
alpha-fluorinated phosphonates as substrate mimics for glucose 6-phosphate dehydrogenase: the CHF stereochemistry matters.J Org Chem. 2000 Jul 28;65(15):4498-508. doi: 10.1021/jo000220v. J Org Chem. 2000. PMID: 10959850
-
Novel 5-arylcarbamoyl-2-methylisoxazolidin-3-yl-3-phosphonates as nucleotide analogues.Nucleosides Nucleotides Nucleic Acids. 2014;33(8):552-82. doi: 10.1080/15257770.2014.909046. Nucleosides Nucleotides Nucleic Acids. 2014. PMID: 25009989
-
Chemical Synthesis of Acyclic Nucleoside Phosphonate Analogs Linked with Cyclic Systems between the Phosphonate and the Base Moieties.Curr Med Chem. 2020;27(35):5918-5948. doi: 10.2174/0929867326666190620100217. Curr Med Chem. 2020. PMID: 31250746 Review.
-
Hydroxy- and Amino-Phosphonates and -Bisphosphonates: Synthetic Methods and Their Biological Applications.Front Chem. 2022 Jun 1;10:890696. doi: 10.3389/fchem.2022.890696. eCollection 2022. Front Chem. 2022. PMID: 35721002 Free PMC article. Review.
Cited by
-
Phosphorylated Nitrones-Synthesis and Applications.Molecules. 2025 Mar 16;30(6):1333. doi: 10.3390/molecules30061333. Molecules. 2025. PMID: 40142107 Free PMC article. Review.
-
Synthesis of Diversely Substituted Diethyl (Pyrrolidin-2-Yl)Phosphonates.Molecules. 2025 May 7;30(9):2078. doi: 10.3390/molecules30092078. Molecules. 2025. PMID: 40363884 Free PMC article.
References
-
- Massiot G., Delaude C. Pyrrolidine Alkaloids. In: Brossi A., editor. The Alkaloids: Chemistry and Pharmacology. Volume 27. Harcourt Brace Jovanovich; Bethesda, MD, USA: 1986. pp. 269–322.
-
- Ali M.S., Ahmad F., Ahmad V.U., Azhar I., Usmanghani K. Unusual Chemical Constituents of Lotus garcinii (Fabaceae) Turk. J. Chem. 2001;25:107–112.
-
- Zhu Y., Wang Y., Gu B.-B., Yang F., Jiao W.-H., Hu G.-H., Yu H.-B., Han B.-N., Zhang W., Shen Y., et al. Antifungal bromopyrrole alkaloids from the South China Sea sponge Agelas sp. Tetrahedron. 2016;72:2964–2971. doi: 10.1016/j.tet.2016.04.020. - DOI
-
- Walsh C.T. Nature loves nitrogen heterocycles. Tetrahedron Lett. 2015;56:3075–3081. doi: 10.1016/j.tetlet.2014.11.046. - DOI
-
- Colegate S.M., Dorling P.R., Huxtable C.R. A Spectroscopic Investigation of Swainsonine: An α-Mannosidase Inhibitor Isolated from Swainsona canescens. Aust. J. Chem. 1979;32:2257–2264. doi: 10.1071/CH9792257. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

