VTC4 Polyphosphate Polymerase Knockout Increases Stress Resistance of Saccharomyces cerevisiae Cells
- PMID: 34070801
- PMCID: PMC8227513
- DOI: 10.3390/biology10060487
VTC4 Polyphosphate Polymerase Knockout Increases Stress Resistance of Saccharomyces cerevisiae Cells
Abstract
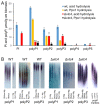
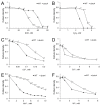
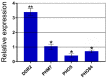
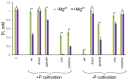
Inorganic polyphosphate (polyP) is an important factor of alkaline, heavy metal, and oxidative stress resistance in microbial cells. In yeast, polyP is synthesized by Vtc4, a subunit of the vacuole transporter chaperone complex. Here, we report reduced but reliably detectable amounts of acid-soluble and acid-insoluble polyPs in the Δvtc4 strain of Saccharomyces cerevisiae, reaching 10% and 20% of the respective levels of the wild-type strain. The Δvtc4 strain has decreased resistance to alkaline stress but, unexpectedly, increased resistance to oxidation and heavy metal excess. We suggest that increased resistance is achieved through elevated expression of DDR2, which is implicated in stress response, and reduced expression of PHO84 encoding a phosphate and divalent metal transporter. The decreased Mg2+-dependent phosphate accumulation in Δvtc4 cells is consistent with reduced expression of PHO84. We discuss a possible role that polyP level plays in cellular signaling of stress response mobilization in yeast.
Keywords: DDR2; PHO84; VTC4; inorganic polyphosphate; oxidative and heavy metal stress; yeast.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
The vtc4 gene influences polyphosphate storage, morphogenesis, and virulence in the maize pathogen Ustilago maydis.Eukaryot Cell. 2006 Aug;5(8):1399-409. doi: 10.1128/EC.00131-06. Eukaryot Cell. 2006. PMID: 16896223 Free PMC article.
-
Ddp1 Cooperates with Ppx1 to Counter a Stress Response Initiated by Nonvacuolar Polyphosphate.mBio. 2022 Aug 30;13(4):e0039022. doi: 10.1128/mbio.00390-22. Epub 2022 Jul 7. mBio. 2022. PMID: 35862758 Free PMC article.
-
The Reduced Level of Inorganic Polyphosphate Mobilizes Antioxidant and Manganese-Resistance Systems in Saccharomyces cerevisiae.Cells. 2019 May 15;8(5):461. doi: 10.3390/cells8050461. Cells. 2019. PMID: 31096715 Free PMC article.
-
Enzymes of yeast polyphosphate metabolism: structure, enzymology and biological roles.Biochem Soc Trans. 2016 Feb;44(1):234-9. doi: 10.1042/BST20150213. Biochem Soc Trans. 2016. PMID: 26862210 Review.
-
Enzymes of Polyphosphate Metabolism in Yeast: Properties, Functions, Practical Significance.Biochemistry (Mosc). 2021 Jan;86(Suppl 1):S96-S108. doi: 10.1134/S0006297921140078. Biochemistry (Mosc). 2021. PMID: 33827402 Review.
Cited by
-
Quantitative proteomic analysis reveals unique Hsp90 cycle-dependent client interactions.Genetics. 2024 Jun 5;227(2):iyae057. doi: 10.1093/genetics/iyae057. Genetics. 2024. PMID: 38606935 Free PMC article.
-
Genetic framework sequencing analysis of Candida tropicalis in dairy cow mastitis and study of pathogenicity and drug resistance.BMC Microbiol. 2024 Oct 23;24(1):428. doi: 10.1186/s12866-024-03522-y. BMC Microbiol. 2024. PMID: 39443857 Free PMC article.
-
The YBR056W-A and Its Ortholog YDR034W-B of S. cerevisiae Belonging to CYSTM Family Participate in Manganese Stress Overcoming.Genes (Basel). 2023 Apr 27;14(5):987. doi: 10.3390/genes14050987. Genes (Basel). 2023. PMID: 37239347 Free PMC article.
-
The PPIP5K Family Member Asp1 Controls Inorganic Polyphosphate Metabolism in S. pombe.J Fungi (Basel). 2021 Jul 31;7(8):626. doi: 10.3390/jof7080626. J Fungi (Basel). 2021. PMID: 34436165 Free PMC article.
-
Multiple effects of the PHO91 gene knockout in Ogataea parapolymorpha.Folia Microbiol (Praha). 2023 Aug;68(4):587-593. doi: 10.1007/s12223-023-01039-x. Epub 2023 Feb 8. Folia Microbiol (Praha). 2023. PMID: 36753030
References
-
- Tiwari P., Gosain T.P., Singh M., Sankhe G.D., Arora G., Kidwai S., Agarwal S., Chugh S., Saini D.K., Singh R. Inorganic polyphosphate accumulation suppresses the dormancy response and virulence in Mycobacterium tuberculosis. J. Biol. Chem. 2019;294:10819–10832. doi: 10.1074/jbc.RA119.008370. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

