Antimicrobial and Antibiofilm Activities of Weissella cibaria against Pathogens of Upper Respiratory Tract Infections
- PMID: 34070813
- PMCID: PMC8229644
- DOI: 10.3390/microorganisms9061181
Antimicrobial and Antibiofilm Activities of Weissella cibaria against Pathogens of Upper Respiratory Tract Infections
Abstract
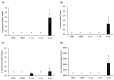
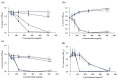
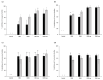
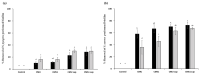
Recently discovered preventive effects of probiotics on oral health have attracted interest to their use for the prevention and treatment of various diseases. This study aimed to evaluate the antimicrobial and antibiofilm properties of Weissella cibaria against Streptococcus pyogenes, Staphylococcus aureus, S. pneumoniae, and Moraxella catarrhalis, the major pathogens of upper respiratory tract infections (URTIs). The antimicrobial activities of W. cibaria were compared with those of other oral probiotics using a competitive inhibition assay and the determination of the minimum inhibitory concentrations (MICs). In addition, a time-kill assay, spectrophotometry, and confocal laser scanning microscopy were used to confirm the antimicrobial and antibiofilm abilities of W. cibaria CMU (oraCMU) and CMS1 (oraCMS1). Both live cells and cell-free supernatants of all tested probiotics, except Streptococcus salivarius, showed excellent antimicrobial activities. All target pathogens were killed within 4 to 24 h at twice the MIC of oraCMU and oraCMS1, which showed the highest antimicrobial activities against M. catarrhalis. The antimicrobial substances that affected different target pathogens were different. Both oraCMU and oraCMS1 showed excellent abilities to inhibit biofilm formation and remove preformed biofilms. Our results suggest that the W. cibaria probiotics offer new possibilities for the prevention and treatment of bacterial URTIs.
Keywords: Weissella cibaria; antibiofilm; antimicrobial; probiotic; upper respiratory tract.
Conflict of interest statement
J.-E.Y., G.-Y.P. and M.-S.K. are employees of OraPharm, Inc. The other authors report no competing financial interests.
Figures



Similar articles
-
The Weissella Genus: Clinically Treatable Bacteria with Antimicrobial/Probiotic Effects on Inflammation and Cancer.Microorganisms. 2022 Dec 7;10(12):2427. doi: 10.3390/microorganisms10122427. Microorganisms. 2022. PMID: 36557680 Free PMC article. Review.
-
Comparative Study on the Characteristics of Weissella cibaria CMU and Probiotic Strains for Oral Care.Molecules. 2016 Dec 20;21(12):1752. doi: 10.3390/molecules21121752. Molecules. 2016. PMID: 27999400 Free PMC article.
-
Probiotic Weissella cibaria displays antibacterial and anti-biofilm effect against cavity-causing Streptococcus mutans.Microb Pathog. 2023 Jul;180:106151. doi: 10.1016/j.micpath.2023.106151. Epub 2023 May 10. Microb Pathog. 2023. PMID: 37172659
-
Safety Evaluation of Oral Care Probiotics Weissella cibaria CMU and CMS1 by Phenotypic |and Genotypic Analysis.Int J Mol Sci. 2019 May 31;20(11):2693. doi: 10.3390/ijms20112693. Int J Mol Sci. 2019. PMID: 31159278 Free PMC article.
-
Weissella: An Emerging Bacterium with Promising Health Benefits.Probiotics Antimicrob Proteins. 2021 Aug;13(4):915-925. doi: 10.1007/s12602-021-09751-1. Epub 2021 Feb 9. Probiotics Antimicrob Proteins. 2021. PMID: 33565028 Review.
Cited by
-
Cross-Over Application of Algerian Dairy Lactic Acid Bacteria for the Design of Plant-Based Products: Characterization of Weissella cibaria and Lactiplantibacillus plantarum for the Formulation of Quinoa-Based Beverage.Microorganisms. 2024 Oct 9;12(10):2042. doi: 10.3390/microorganisms12102042. Microorganisms. 2024. PMID: 39458351 Free PMC article.
-
Antibacterial activity of medicinal plants in Indonesia on Streptococcus pneumoniae.PLoS One. 2022 Sep 13;17(9):e0274174. doi: 10.1371/journal.pone.0274174. eCollection 2022. PLoS One. 2022. PMID: 36099236 Free PMC article.
-
In Vitro Evaluation of Probiotic Properties and Anti-Pathogenic Effects of Lactobacillus and Bifidobacterium Strains as Potential Probiotics.Foods. 2024 Jul 22;13(14):2301. doi: 10.3390/foods13142301. Foods. 2024. PMID: 39063385 Free PMC article.
-
Protective Effect of [Cu(NN1)2](ClO4) Complex in Rainbow Trout Challenged against Flavobacterium psychrophilum.Microorganisms. 2022 Nov 19;10(11):2296. doi: 10.3390/microorganisms10112296. Microorganisms. 2022. PMID: 36422366 Free PMC article.
-
The Weissella Genus: Clinically Treatable Bacteria with Antimicrobial/Probiotic Effects on Inflammation and Cancer.Microorganisms. 2022 Dec 7;10(12):2427. doi: 10.3390/microorganisms10122427. Microorganisms. 2022. PMID: 36557680 Free PMC article. Review.
References
-
- Johnston S., Holgate S. Epidemiology of viral respiratory infections. In: Myint S., Taylor-Robinson D., editors. Viral and Other Infections of the Human Respiratory Tract. Springer; Dordrecht, The Netherlands: 1996.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

