Evaluation of a Pseudovirus Neutralization Assay for SARS-CoV-2 and Correlation with Live Virus-Based Micro Neutralization Assay
- PMID: 34070824
- PMCID: PMC8226551
- DOI: 10.3390/diagnostics11060994
Evaluation of a Pseudovirus Neutralization Assay for SARS-CoV-2 and Correlation with Live Virus-Based Micro Neutralization Assay
Abstract
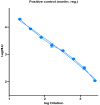
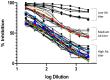
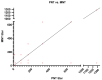
The unusual cases of pneumonia outbreak were reported from Wuhan city in late December 2019. Serological testing provides a powerful tool for the identification of prior infection and for epidemiological studies. Pseudotype virus neutralization assays are widely used for many viruses and applications in the fields of serology. The accuracy of pseudotype neutralizing assay allows for its use in low biosafety lab and provides a safe and effective alternative to the use of wild-type viruses. In this study, we evaluated the performance of this assay compared to the standard microneutralization assay as a reference. The lentiviral pseudotype particles were generated harboring the Spike gene of SARS-CoV-2. The generated pseudotype particles assay was used to evaluate the activity of neutralizing antibodies in 300 human serum samples from a COVID-19 sero-epidemiological study. Testing of these samples resulted in 55 positive samples and 245 negative samples by pseudotype viral particles assay while microneutralization assay resulted in 64 positive and 236 negative by MN assay. Compared to the MN, the pseudotyped viral particles assay showed a sensitivity of 85.94% and a specificity of 100%. Based on the data generated from this study, the pseudotype-based neutralization assay showed a reliable performance for the detection of neutralizing antibodies against SARS-CoV-2 and can be used safely and efficiently as a diagnostic tool in a biosafety level 2 laboratory.
Keywords: SARS-CoV-2; Saudi Arabia; coronavirus; micro-neutralization assay; pseudotype.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Gorbalenya A.E. Severe acute respiratory syndrome-related coronavirus–The species and its viruses, a statement of the Coronavirus Study Group. BioRxiv. 2020:937862v1. doi: 10.1101/2020.02.07. - DOI
-
- [(accessed on 25 May 2021)]; Available online: https://covid19.who.int/
-
- Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S., Yuen K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

