Restoring the Oxidase-Like Activity of His@AuNCs for the Determination of Alkaline Phosphatase
- PMID: 34070918
- PMCID: PMC8227771
- DOI: 10.3390/bios11060174
Restoring the Oxidase-Like Activity of His@AuNCs for the Determination of Alkaline Phosphatase
Abstract
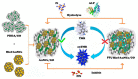
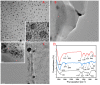
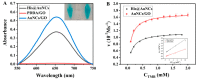
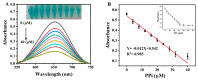
In this paper, we propose a simple colorimetric method for the sensitive and selective detection of alkaline phosphatase (ALP) activity based on the turn off/turn on oxidase mimic activity of His@AuNCs. His@AuNCs/graphene oxide hybrids (His@AuNCs/GO) were easily obtained using the self-assembly method with poly (diallyldimethylammonium chloride) (PDDA)-coated GO and showed high oxidase-like activity compared with His@AuNCs. We found that the pyrophosphate ion (P2O74-, PPi) could effectively inhibit the oxidase mimic activity of His@AuNCs/GO, and the hydrolysis of PPi by ALP restored the inhibited activity of His@AuNCs/GO, enabling them to efficiently catalyze the oxidation of 3,3',5,5'-tetramethylbenzidine (TMB) to generate the blue oxidized product oxTMB. The intensity of the color showed a linear dependency with the ALP activity. ALP was detected in the linear range of 0-40 mU/mL with a low detection limit (LOD) of 0.26 mU/mL (S/N = 3). The proposed method is fast, easy, and can be applied to monitor the ALP activity in serum samples accurately and effectively, which suggests its practicability and reliability in the detection of ALP activity in clinical practice.
Keywords: ALP; His@AuNCs/GO; PPi; colorimetric detection; oxidase-like activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Spectrophotometric determination of the activity of alkaline phosphatase and detection of its inhibitors by exploiting the pyrophosphate-accelerated oxidase-like activity of nanoceria.Mikrochim Acta. 2019 May 2;186(5):320. doi: 10.1007/s00604-019-3423-8. Mikrochim Acta. 2019. PMID: 31049712
-
Colorimetric determination of the activity of alkaline phosphatase by exploiting the oxidase-like activity of palladium cube@CeO2 core-shell nanoparticles.Mikrochim Acta. 2020 Jan 9;187(2):115. doi: 10.1007/s00604-019-4070-9. Mikrochim Acta. 2020. PMID: 31919598
-
Ratiometric fluorescent sensor for visual determination of copper ions and alkaline phosphatase based on carbon quantum dots and gold nanoclusters.Anal Bioanal Chem. 2019 May;411(12):2531-2543. doi: 10.1007/s00216-019-01693-6. Epub 2019 Mar 4. Anal Bioanal Chem. 2019. PMID: 30828757
-
Enhanced His@AuNCs oxidase-like activity by reduced graphene oxide and its application for colorimetric and electrochemical detection of nitrite.Anal Bioanal Chem. 2019 Apr;411(10):2189-2200. doi: 10.1007/s00216-019-01655-y. Epub 2019 Mar 13. Anal Bioanal Chem. 2019. PMID: 30868189
-
Sensitive and selective colorimetric detection of alkaline phosphatase activity based on phosphate anion-quenched oxidase-mimicking activity of Ce(Ⅳ) ions.Anal Chim Acta. 2018 Dec 31;1044:154-161. doi: 10.1016/j.aca.2018.09.045. Epub 2018 Sep 20. Anal Chim Acta. 2018. PMID: 30442397
Cited by
-
Colorimetric detection of alkaline phosphatase based on the off-on effect of light-responsive oxidase mimicking activity of covalent organic framework (Cu-TpBpy-COF) under near-neutral condition.Mikrochim Acta. 2024 Jan 13;191(2):93. doi: 10.1007/s00604-023-06128-9. Mikrochim Acta. 2024. PMID: 38217686
-
A turn-on chemiluminescent assay for alkaline phosphatase using two-dimensional Fe-centered metal-organic frameworks as the signaling probe.Anal Sci. 2023 Sep;39(9):1541-1550. doi: 10.1007/s44211-023-00370-0. Epub 2023 May 25. Anal Sci. 2023. PMID: 37227624
References
-
- Fernley H.N. Enzymes. Academic Press; Cambridge, MA, USA: 1971. 18 Mammalian Alkaline Phosphatases; pp. 417–447. - DOI
-
- Couttenye M.M., D’Haese P.C., Van H.V.O., Lemoniatou E., Goodman W., Verpooten G.A., De B.M.E. Low serum levels of alkaline phosphatase of bone origin: A good marker of adynamic bone disease in haemodialysis patients. Nephrol. Dial. Transplant. 1996;11:1065–1072. doi: 10.1093/oxfordjournals.ndt.a027457. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

