Biomarkers in Hepatobiliary Cancers: What is Useful in Clinical Practice?
- PMID: 34070929
- PMCID: PMC8198554
- DOI: 10.3390/cancers13112708
Biomarkers in Hepatobiliary Cancers: What is Useful in Clinical Practice?
Abstract
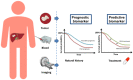
Hepatocellular carcinoma (HCC) and biliary tract cancers (BTC) exhibit a poor prognosis with 5-year overall survival rates around 15%, all stages combined. Most of these primary liver malignancies are metastatic at diagnostic, with only limited therapeutic options, relying mainly on systemic therapies. Treatment modalities are different yet partially overlapping between HCC and BTC. The complex molecular profile of BTC yields to several actionable therapeutic targets, contrary to HCC that remains the field of antiangiogenic drugs in non-molecularly selected patients. Immunotherapy is now validated in the first line in HCC in combination with bevacizumab, while clinical activity of single agent immunotherapy appears limited to a subset of patients in BTC, still poorly characterized, and combinations are currently under investigation. In this review, we provide a critical evaluation and grading of clinical relevance on (i) the main prognostic biomarkers in HCC and BTC, (ii) the main theragnostic biomarkers in both tumors, and lastly (iii) what is recommended in clinical practice.
Keywords: biliary tract cancers; biomarker; cholangiocarcinoma; hepatocellular carcinoma; immune checkpoint inhibitor; targeted therapy.
Conflict of interest statement
Alice Boilève declares no conflicts of interest. Marc Hilmi declares no conflicts of interest. Matthieu Delaye declares no conflicts of interest. Annemilaï Raballand declares no conflicts of interest. Cindy Neuzillet declares conflicts of interest: consultancy/honoraria: Pierre Fabre, Servier, Roche, AstraZeneca, Bristol-Myers Squibb, Amgen, Merck, MSD, Novartis, Incyte Biosciences, Mylan, Baxter, Nutricia, Fresenius Kabi, Kiplin; Research funding: Roche, Merck; Clinical trials: OSE Immunotherapeutics, AstraZeneca, Bristol-Myers Squibb.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

