HPV Strain Predicts Severity of Juvenile-Onset Recurrent Respiratory Papillomatosis with Implications for Disease Screening
- PMID: 34070981
- PMCID: PMC8197133
- DOI: 10.3390/cancers13112556
HPV Strain Predicts Severity of Juvenile-Onset Recurrent Respiratory Papillomatosis with Implications for Disease Screening
Abstract
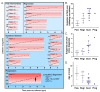
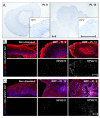
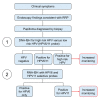
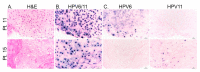
Juvenile-onset recurrent respiratory papillomatosis (JoRRP) is the most common benign neoplasm of the larynx in children, presenting with significant variation in clinical course and potential for progression to malignancy. Since JoRRP is driven by human papillomavirus (HPV), we evaluated viral factors in a prospective cohort to identify predictive factors of disease severity. Twenty children with JoRRP undergoing routine debridement of papillomas were recruited and followed for ≥1 year. Demographical features, clinical severity scores, and surgeries over time were tabulated. Biopsies were used to establish a tissue bank and primary cell cultures for HPV6 vs. HPV11 genotyping and evaluation of viral gene expression. We found that patients with HPV11+ disease had an earlier age at disease onset, higher frequency of surgeries, increased number of lifetime surgeries, and were more likely to progress to malignancy. However, the amplitude of viral E6/E7 gene expression did not account for increased disease severity in HPV11+ patients. Determination of HPV strain is not routinely performed in the standard of care for JoRRP patients; we demonstrate the utility and feasibility of HPV genotyping using RNA-ISH for screening of HPV11+ disease as a biomarker for disease severity and progression in JoRRP patients.
Keywords: HPV strain; JoRRP; RRP; juvenile-onset recurrent respiratory papillomatosis; laryngeal papillomas; low-risk HPV.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

