Evaluation of the Efficacy of Lacer HaliTM Treatment on the Management of Halitosis: A Randomized Double-Blind Clinical Trial
- PMID: 34071005
- PMCID: PMC8197132
- DOI: 10.3390/jcm10112256
Evaluation of the Efficacy of Lacer HaliTM Treatment on the Management of Halitosis: A Randomized Double-Blind Clinical Trial
Abstract
Background: Halitosis of oral origin is very common in the general population. Due to their antimicrobial properties, chlorhexidine-based products are widely used in the management of this condition, but these are associated with reversible side effects. In this study we evaluated the efficacy of Lacer HaliTM mouthrinse and toothpaste in subjects with intraoral halitosis after several applications under normal conditions of use.
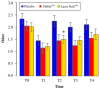
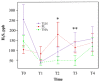
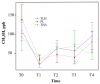
Methods: In this randomized clinical trial with mouth rinse and toothpaste, single-center, double-blinded, parallel participants were assigned to an experimental group (Lacer HaliTM,, n = 20), a positive control group (HalitaTM, n = 20), and a placebo group (n = 20). The active duration of the study was 18 days. The clinical follow-up evaluations were performed at five time points (T0, T1, T2, T3, and T4). The intensity of halitosis was evaluated by organoleptic measurement and the portable gas chromatograph OralChromaTM. The data were analyzed using generalized mixed linear models.
Results: Sixty patients completed the study. Lacer HaliTM, in comparison with HalitaTM, did not show statistically significant differences at any time during the study except for the levels of hydrogen sulfide and total volatile sulfur compounds at 15 days, where HalitaTM was better. Compared to the placebo treatment, Lacer HaliTM, was significantly more efficient, in terms of both the organoleptic evaluations at 8 days and the levels of hydrogen sulfide.
Conclusions: Lacer HaliTM is an alternative to chlorhexidine-based toothpaste and mouthwashes in the management of halitosis.
Keywords: Lacer HaliTM; OralChromaTM; halitosis; octenidine; oral malodor; organoleptic score.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Effect of Halita mouthwash on oral halitosis treatment: A randomized triple-blind clinical trial.J Dent Res Dent Clin Dent Prospects. 2019 Winter;13(1):31-35. Epub 2019 Apr 24. J Dent Res Dent Clin Dent Prospects. 2019. PMID: 31217916 Free PMC article.
-
The OralChromaTM CHM-2: a comparison with the OralChromaTM CHM-1.Clin Oral Investig. 2020 Aug;24(8):2829-2836. doi: 10.1007/s00784-019-03148-9. Epub 2020 Jan 16. Clin Oral Investig. 2020. PMID: 31950293
-
Clinical effects of a new mouthrinse containing chlorhexidine, cetylpyridinium chloride and zinc-lactate on oral halitosis. A dual-center, double-blind placebo-controlled study.J Clin Periodontol. 2003 Apr;30(4):300-6. doi: 10.1034/j.1600-051x.2003.00342.x. J Clin Periodontol. 2003. PMID: 12694427 Clinical Trial.
-
Halitosis: A frequently ignored social condition.J Int Soc Prev Community Dent. 2011 Jan;1(1):9-13. doi: 10.4103/2231-0762.86374. J Int Soc Prev Community Dent. 2011. PMID: 24478947 Free PMC article. Review.
-
[Halitosis in 1999].Rev Stomatol Chir Maxillofac. 1999 Oct;100(5):240-4. Rev Stomatol Chir Maxillofac. 1999. PMID: 10604216 Review. French.
Cited by
-
Managing halitosis during the SARS-CoV-2 pandemic.J Dent Sci. 2022 Jul;17(3):1418-1419. doi: 10.1016/j.jds.2022.04.020. Epub 2022 May 2. J Dent Sci. 2022. PMID: 35530437 Free PMC article. No abstract available.
References
-
- Porter S.R., Scully C. Oral malodour (halitosis) Br. Med. J. 2006;333:632–635. doi: 10.1136/bmj.38954.631968.AE. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

