Pyelonephritis in Pediatric Uropathic Patients: Differences from Community-Acquired Ones and Therapeutic Protocol Considerations. A 10-Year Single-Center Retrospective Study
- PMID: 34071019
- PMCID: PMC8224700
- DOI: 10.3390/children8060436
Pyelonephritis in Pediatric Uropathic Patients: Differences from Community-Acquired Ones and Therapeutic Protocol Considerations. A 10-Year Single-Center Retrospective Study
Abstract
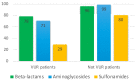
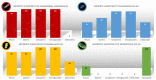
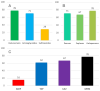
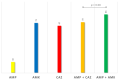
Pyelonephritis (PN) represents an important cause of morbidity in the pediatric population, especially in uropathic patients. The aim of the study is to demonstrate differences between PNs of uropathic patients and PNs acquired in community in terms of uropathogens involved and antibiotic sensitivity; moreover, to identify a proper empiric therapeutic strategy. A retrospective study was conducted on antibiograms on urine cultures from PNs in vesicoureteral reflux (VUR) patients admitted to pediatric surgery department and from PNs in not VUR patients admitted to Pediatric Emergency Unit between 2010 and 2020. We recorded 58 PNs in 33 patients affected by VUR and 112 PNs in the not VUR group. The mean age of not VUR patients at the PN episode was 1.3 ± 2.6 years (range: 20 days of life-3 years), and almost all the urine cultures, 111 (99.1%), isolated Gram-negative bacteria and rarely, 1 (0.9%), Gram-positive bacteria. The Gram-negative uropathogens isolated were Escherichia coli (97%), Proteus mirabilis (2%), and Klebsiella spp. (1%). The only Gram-positive bacteria isolated was an Enterococcus faecalis. As regards the antibiograms, 96% of not VUR PNs responded to beta-lactams, 99% to aminoglycosides, and 80% to sulfonamides. For the VUR group, mean age was 3.0 years ± 3.0 years (range: 9 days of life-11 years) and mean number of episodes per patient was 2.0 ± 1.0 (range: 1-5); 83% of PNs were by Gram-negatives bacteria vs. 17% by Gram-positive: the most important Gram-negative bacteria were Pseudomonas aeruginosa (44%), Escherichia coli (27%), and Klebsiella spp. (12%), while Enterococcus spp. determined 90% of Gram-positive UTIs. Regimen ampicillin/ceftazidime (success rate: 72.0%) was compared to ampicillin/amikacin (success rate of 83.0%): no statistically significant difference was found (p = 0.09). The pathogens of PNs in uropathic patients are different from those of community-acquired PNs, and clinicians should be aware of their peculiar antibiotic susceptibility. An empiric therapy based on the association ampicillin + ceftazidime is therefore suggested.
Keywords: Pediatric Urology; antibiotic resistant; antibiotic therapy; pyelonephritis; uropathogens; vesicoureteral reflux.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

