Massachusetts' Findings from Statewide Newborn Screening for Spinal Muscular Atrophy
- PMID: 34071063
- PMCID: PMC8162354
- DOI: 10.3390/ijns7020026
Massachusetts' Findings from Statewide Newborn Screening for Spinal Muscular Atrophy
Abstract
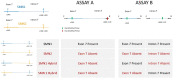
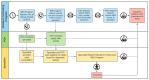
Massachusetts began newborn screening (NBS) for Spinal Muscular Atrophy (SMA) following the availability of new treatment options. The New England Newborn Screening Program developed, validated, and implemented a screening algorithm for the detection of SMA-affected infants who show absent SMN1 Exon 7 by Real-Time™ quantitative PCR (qPCR). We screened 179,467 neonates and identified 9 SMA-affected infants, all of whom were referred to a specialist by day of life 6 (average and median 4 days of life). Another ten SMN1 hybrids were observed but never referred. The nine referred infants who were confirmed to have SMA were entered into treatment protocols. Early data show that some SMA-affected children have remained asymptomatic and are meeting developmental milestones and some have mild to moderate delays. The Massachusetts experience demonstrates that SMA NBS is feasible, can be implemented on a population basis, and helps engage infants for early treatment to maximize benefit.
Keywords: SMN1 gene; SMN2 gene; Spinal Muscular Atrophy; newborn screening.
Conflict of interest statement
B.T.D. has served as an ad hoc scientific advisory board member for Audentes, AveXis, Biogen, Pfizer, Vertex, PTC and Sarepta; Steering Committee Chair for Roche and DSMB member for Amicus Inc.; he has no financial interests in these companies. B.T.D. has received research support from the National Institutes of Health/National Institute of Neurological Disorders and Stroke, the Slaney Family Fund for SMA, the Spinal Muscular Atrophy Foundation, CureSMA, and Working on Walking Fund and has received grants from Ionis Pharmaceuticals, Inc., for the ENDEAR, CHERISH, CS2/CS12 studies; from Biogen for CS11; and from AveXis, Cytokinetics, Sarepta Pharmaceuticals, PTC Therapeutics, Roche, Santhera, Scholar Rock, Fibrogen, and Summit. K.J.S. has served as an ad hoc scientific advisory board member for Cure SMA, Ionis, Biogen, Roche and Novartis without compensation or financial interests. She has received grant funding from NIH/NICHD and Biogen, and clinical trial support from Ionis, Biogen and AveXis (Novartis).
Figures

References
-
- Advisory Committee on Heritable Disorders in Newborns and Children. [(accessed on 19 March 2021)]; Available online: https://www.hrsa.gov/sites/default/files/hrsa/advisory-committees/herita....
-
- NewSTEPs: A Program of the Association of Public Health Laboratories. [(accessed on 19 March 2021)]; Available online: https://www.newsteps.org/resources/newborn-screening-status-all-disorders.
-
- Taylor J.L., Lee F.K., Yazdanpanah G.K., Staropoli J.F., Liu M., Carulli J.P., Sun C., Dobrowolski S.F., Hannon W.H., Vogt R.F. Newborn blood spot screening test using multiplexed real-time PCR to simultaneously screen for spinal muscular atrophy and severe combined immunodeficiency. Clin. Chem. 2015;61:412–419. doi: 10.1373/clinchem.2014.231019. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

