Modulation of Bovine Endometrial Cell Receptors and Signaling Pathways as a Nanotherapeutic Exploration against Dairy Cow Postpartum Endometritis
- PMID: 34071093
- PMCID: PMC8224678
- DOI: 10.3390/ani11061516
Modulation of Bovine Endometrial Cell Receptors and Signaling Pathways as a Nanotherapeutic Exploration against Dairy Cow Postpartum Endometritis
Abstract
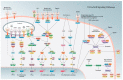
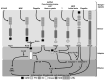
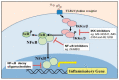
In order to control and prevent bovine endometritis, there is a need to understand the molecular pathogenesis of the infectious disease. Bovine endometrium is usually invaded by a massive mobilization of microorganisms, especially bacteria, during postpartum dairy cows. Several reports have implicated the Gram-negative bacteria in the pathogenesis of bovine endometritis, with information dearth on the potentials of Gram-positive bacteria and their endotoxins. The invasive bacteria and their ligands pass through cellular receptors such as TLRs, NLRs, and biomolecular proteins of cells activate the specific receptors, which spontaneously stimulates cellular signaling pathways like MAPK, NF-kB and sequentially triggers upregulation of pro-inflammatory cytokines. The cascade of inflammatory induction involves a dual signaling pathway; the transcription factor NF-κB is released from its inhibitory molecule and can bind to various inflammatory genes promoter. The MAPK pathways are concomitantly activated, leading to specific phosphorylation of the NF-κB. The provision of detailed information on the molecular pathomechanism of bovine endometritis with the interaction between host endometrial cells and invasive bacteria in this review would widen the gap of exploring the potential of receptors and signal transduction pathways in nanotechnology-based drug delivery system. The nanotherapeutic discovery of endometrial cell receptors, signal transduction pathway, and cell biomolecules inhibitors could be developed for strategic inhibition of infectious signals at the various cell receptors and signal transduction levels, interfering on transcription factors activation and pro-inflammatory cytokines and genes expression, which may significantly protect endometrium against postpartum microbial invasion.
Keywords: PAMP; biomolecules; cell receptors; cytokines; dairy cow; endometritis; nanosystem; nanotherapy; signaling pathways.
Conflict of interest statement
The authors declare they have no competing interests.
Figures
Similar articles
-
Cortisol inhibits NF-κB and MAPK pathways in LPS activated bovine endometrial epithelial cells.Int Immunopharmacol. 2018 Mar;56:71-77. doi: 10.1016/j.intimp.2018.01.021. Epub 2018 Jan 23. Int Immunopharmacol. 2018. PMID: 29367089
-
Bovine Endometrial Epithelial Cells Scale Their Pro-inflammatory Response In vitro to Pathogenic Trueperella pyogenes Isolated from the Bovine Uterus in a Strain-Specific Manner.Front Cell Infect Microbiol. 2017 Jun 21;7:264. doi: 10.3389/fcimb.2017.00264. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28680854 Free PMC article.
-
Integrated analysis of the local and systemic changes preceding the development of post-partum cytological endometritis.BMC Genomics. 2015 Oct 19;16:811. doi: 10.1186/s12864-015-1967-5. BMC Genomics. 2015. PMID: 26482908 Free PMC article.
-
MicroRNAome: Potential and Veritable Immunomolecular Therapeutic and Diagnostic Baseline for Lingering Bovine Endometritis.Front Vet Sci. 2020 Dec 23;7:614054. doi: 10.3389/fvets.2020.614054. eCollection 2020. Front Vet Sci. 2020. PMID: 33426032 Free PMC article. Review.
-
A review of the ongoing discussion about definition, diagnosis and pathomechanism of subclinical endometritis in dairy cows.Theriogenology. 2017 May;94:21-30. doi: 10.1016/j.theriogenology.2017.02.005. Epub 2017 Feb 12. Theriogenology. 2017. PMID: 28407857 Review.
Cited by
-
MicroRNA miR-24-3p Mediates the Negative Regulation of Lipopolysaccharide-Induced Endometrial Inflammatory Response by Targeting TNF Receptor-Associated Factor 6 (TRAF6).J Inflamm Res. 2022 Feb 6;15:807-825. doi: 10.2147/JIR.S347293. eCollection 2022. J Inflamm Res. 2022. PMID: 35173455 Free PMC article.
-
An Overview of Bioactive Compounds' Role in Modulating the Nrf2/Keap1/NF-κB Pathway to Alleviate Lipopolysaccharide-Induced Endometritis.Int J Mol Sci. 2024 Sep 25;25(19):10319. doi: 10.3390/ijms251910319. Int J Mol Sci. 2024. PMID: 39408650 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

