A Single-Domain Antibody-Based Anti-PSMA Recombinant Immunotoxin Exhibits Specificity and Efficacy for Prostate Cancer Therapy
- PMID: 34071152
- PMCID: PMC8197099
- DOI: 10.3390/ijms22115501
A Single-Domain Antibody-Based Anti-PSMA Recombinant Immunotoxin Exhibits Specificity and Efficacy for Prostate Cancer Therapy
Abstract
Prostate cancer (PCa) is the second most common cancer in men, causing more than 300,000 deaths every year worldwide. Due to their superior cell-killing ability and the relative simplicity of their preparation, immunotoxin molecules have great potential in the clinical treatment of cancer, and several such molecules have been approved for clinical application. In this study, we adopted a relatively simple strategy based on a single-domain antibody (sdAb) and an improved Pseudomonas exotoxin A (PE) toxin (PE24X7) to prepare a safer immunotoxin against prostate-specific membrane antigen (PSMA) for PCa treatment. The designed anti-PSMA immunotoxin, JVM-PE24X7, was conveniently prepared in its soluble form in an Escherichia coli (E. coli) system, avoiding the complex renaturation process needed for immunotoxin preparation by the conventional strategy. The product was very stable and showed a very strong ability to bind the PSMA receptor. Cytotoxicity assays showed that this molecule at a very low concentration could kill PSMA-positive PCa cells, with an EC50 value (concentration at which the cell viability decreased by 50%) of 15.3 pM against PSMA-positive LNCaP cells. Moreover, this molecule showed very good killing selectivity between PSMA-positive and PSMA-negative cells, with a selection ratio of more than 300-fold. Animal studies showed that this molecule at a very low dosage (5 × 0.5 mg/kg once every three days) completely inhibited the growth of PCa tumors, and the maximum tolerable dose (MTD) was more than 15 mg/kg, indicating its very potent tumor-treatment ability and a wide therapeutic window. Use of the new PE toxin, PE24X7, as the effector moiety significantly reduced off-target toxicity and improved the therapeutic window of the immunotoxin. The above results demonstrate that the designed anti-PSMA immunotoxin, JVM-PE24X7, has good application value for the treatment of PCa.
Keywords: PE; PSMA (prostate-specific membrane antigen); immunotoxins; prostate carcinoma; sdAbs (single-domain antibodies).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

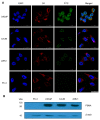


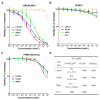
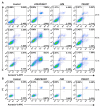

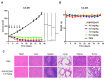
Similar articles
-
Preclinical evaluation of a novel anti-mesothelin immunotoxin based on a single domain antibody as the targeting ligand.Int J Pharm. 2021 Jun 1;602:120647. doi: 10.1016/j.ijpharm.2021.120647. Epub 2021 Apr 26. Int J Pharm. 2021. PMID: 33915185
-
Anti-tumor effects and lack of side effects in mice of an immunotoxin directed against human and mouse prostate-specific membrane antigen.Prostate. 2004 Sep 15;61(1):1-11. doi: 10.1002/pros.20074. Prostate. 2004. PMID: 15287089
-
Preclinical evaluation of a recombinant anti-prostate specific membrane antigen single-chain immunotoxin against prostate cancer.J Immunother. 2010 Apr;33(3):262-71. doi: 10.1097/CJI.0b013e3181c5495c. J Immunother. 2010. PMID: 20445346
-
Efficacy Against Human Prostate Cancer by Prostate-specific Membrane Antigen-specific, Transforming Growth Factor-β Insensitive Genetically Targeted CD8+ T-cells Derived from Patients with Metastatic Castrate-resistant Disease.Eur Urol. 2018 May;73(5):648-652. doi: 10.1016/j.eururo.2017.12.008. Epub 2017 Dec 21. Eur Urol. 2018. PMID: 29275833 Free PMC article. Review.
-
Prostate-Specific Membrane Antigen-Directed Therapy for Metastatic Castration-Resistant Prostate Cancer.Cancer J. 2016 Sep/Oct;22(5):347-352. doi: 10.1097/PPO.0000000000000221. Cancer J. 2016. PMID: 27749329 Free PMC article. Review.
Cited by
-
Nanobody-Based EGFR-Targeting Immunotoxins for Colorectal Cancer Treatment.Biomolecules. 2023 Jun 26;13(7):1042. doi: 10.3390/biom13071042. Biomolecules. 2023. PMID: 37509078 Free PMC article.
-
Multimodal Radiobioconjugates of Magnetic Nanoparticles Labeled with 44Sc and 47Sc for Theranostic Application.Pharmaceutics. 2023 Mar 5;15(3):850. doi: 10.3390/pharmaceutics15030850. Pharmaceutics. 2023. PMID: 36986710 Free PMC article.
-
Gamma radiation coupled ADP-ribosyl transferase activity of Pseudomonas aeruginosa PE24 moiety.Appl Microbiol Biotechnol. 2023 Mar;107(5-6):1765-1784. doi: 10.1007/s00253-023-12401-x. Epub 2023 Feb 18. Appl Microbiol Biotechnol. 2023. PMID: 36808279 Free PMC article.
-
Engineered antibody fusion proteins for targeted disease therapy.Trends Pharmacol Sci. 2021 Dec;42(12):1064-1081. doi: 10.1016/j.tips.2021.09.009. Epub 2021 Oct 25. Trends Pharmacol Sci. 2021. PMID: 34706833 Free PMC article. Review.
-
Nanobodies: a new potential for prostate cancer treatment.J Cancer Res Clin Oncol. 2023 Aug;149(9):6703-6710. doi: 10.1007/s00432-022-04515-y. Epub 2023 Jan 21. J Cancer Res Clin Oncol. 2023. PMID: 36680579 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

