The Non-Coding RNA Landscape in IgA Nephropathy-Where Are We in 2021?
- PMID: 34071162
- PMCID: PMC8198207
- DOI: 10.3390/jcm10112369
The Non-Coding RNA Landscape in IgA Nephropathy-Where Are We in 2021?
Abstract
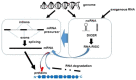
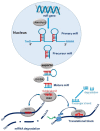
IgA nephropathy (IgAN) is the most commonly diagnosed primary glomerulonephritis worldwide. It is a slow progressing disease with approximately 30% of cases reaching end-stage kidney disease within 20 years of diagnosis. It is currently only diagnosed by an invasive biopsy and treatment options are limited. However, the current surge in interest in RNA interference is opening up new horizons for the use of this new technology in the field of IgAN management. A greater understanding of the fundamentals of RNA interference offers exciting possibilities both for biomarker discovery and, more importantly, for novel therapeutic approaches to target key pathogenic pathways in IgAN. This review aims to summarise the RNA interference literature in the context of microRNAs and their association with the multifaceted aspects of IgA nephropathy.
Keywords: IgA nephropathy; RNA interference; microRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
MicroRNAs: a new avenue to understand, investigate and treat immunoglobulin A nephropathy?Clin Kidney J. 2018 Feb;11(1):29-37. doi: 10.1093/ckj/sfx096. Epub 2017 Sep 23. Clin Kidney J. 2018. PMID: 29423198 Free PMC article.
-
The Emerging Role of Complement Proteins as a Target for Therapy of IgA Nephropathy.Front Immunol. 2019 Mar 19;10:504. doi: 10.3389/fimmu.2019.00504. eCollection 2019. Front Immunol. 2019. PMID: 30941137 Free PMC article. Review.
-
Update on treatment of immunoglobulin A nephropathy.Nephrology (Carlton). 2018 Oct;23 Suppl 4:62-67. doi: 10.1111/nep.13453. Nephrology (Carlton). 2018. PMID: 30298661 Review.
-
Differential expression of urinary exosomal microRNAs in IgA nephropathy.J Clin Lab Anal. 2018 Feb;32(2):e22226. doi: 10.1002/jcla.22226. Epub 2017 Apr 6. J Clin Lab Anal. 2018. PMID: 28383146 Free PMC article.
-
High serum IgA/C3 ratio better predicts a diagnosis of IgA nephropathy among primary glomerular nephropathy patients with proteinuria ≤ 1 g/d: an observational cross-sectional study.BMC Nephrol. 2019 Apr 30;20(1):150. doi: 10.1186/s12882-019-1331-0. BMC Nephrol. 2019. PMID: 31039758 Free PMC article.
Cited by
-
B-Cell Epigenetic Modulation of IgA Response by 5-Azacytidine and IgA Nephropathy.J Am Soc Nephrol. 2024 Dec 1;35(12):1686-1701. doi: 10.1681/ASN.0000000000000441. Epub 2024 Aug 13. J Am Soc Nephrol. 2024. PMID: 39137052
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

