Fish Sidestream-Derived Protein Hydrolysates Suppress DSS-Induced Colitis by Modulating Intestinal Inflammation in Mice
- PMID: 34071180
- PMCID: PMC8228426
- DOI: 10.3390/md19060312
Fish Sidestream-Derived Protein Hydrolysates Suppress DSS-Induced Colitis by Modulating Intestinal Inflammation in Mice
Abstract
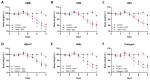
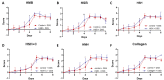
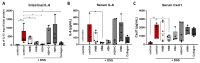
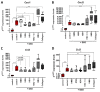
Inflammatory bowel disease is characterized by extensive intestinal inflammation, and therapies against the disease target suppression of the inflammatory cascade. Nutrition has been closely linked to the development and suppression of inflammatory bowel disease, which to a large extent is attributed to the complex immunomodulatory properties of nutrients. Diets containing fish have been suggested to promote health and suppress inflammatory diseases. Even though most of the health-promoting properties of fish-derived nutrients are attributed to fish oil, the potential health-promoting properties of fish protein have not been investigated. Fish sidestreams contain large amounts of proteins, currently unexploited, with potential anti-inflammatory properties, and may possess additional benefits through bioactive peptides and free amino acids. In this project, we utilized fish protein hydrolysates, based on mackerel and salmon heads and backbones, as well as flounder skin collagen. Mice fed with a diet supplemented with different fish sidestream-derived protein hydrolysates (5% w/w) were exposed to the model of DSS-induced colitis. The results show that dietary supplements containing protein hydrolysates from salmon heads suppressed chemically-induced colitis development as determined by colon length and pro-inflammatory cytokine production. To evaluate colitis severity, we measured the expression of different pro-inflammatory cytokines and chemokines and found that the same supplement suppressed the pro-inflammatory cytokines IL-6 and TNFα and the chemokines Cxcl1 and Ccl3. We also assessed the levels of the anti-inflammatory cytokines IL-10 and Tgfb and found that selected protein hydrolysates induced their expression. Our findings demonstrate that protein hydrolysates derived from fish sidestreams possess anti-inflammatory properties in the model of DSS-induced colitis, providing a novel underexplored source of health-promoting dietary supplements.
Keywords: IL-10; IL-6; chemokines; colitis; cytokines; fish protein hydrolysates; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Collagen-Containing Fish Sidestream-Derived Protein Hydrolysates Support Skin Repair via Chemokine Induction.Mar Drugs. 2021 Jul 15;19(7):396. doi: 10.3390/md19070396. Mar Drugs. 2021. PMID: 34356821 Free PMC article.
-
Sturgeon hydrolysates alleviate DSS-induced colon colitis in mice by modulating NF-κB, MAPK, and microbiota composition.Food Funct. 2020 Aug 1;11(8):6987-6999. doi: 10.1039/c9fo02772f. Epub 2020 Jul 23. Food Funct. 2020. PMID: 32701080
-
A soybean and fish oil mixture with different n-6/n-3 PUFA ratios modulates the inflammatory reaction in mice with dextran sulfate sodium-induced acute colitis.Clin Nutr. 2015 Oct;34(5):1018-24. doi: 10.1016/j.clnu.2014.11.008. Epub 2014 Nov 18. Clin Nutr. 2015. PMID: 25434577
-
Atlantic salmon (Salmo salar) waste as a unique source of biofunctional protein hydrolysates: Emerging productions, promising applications, and challenges mitigation.Food Chem. 2025 Jan 1;462:141017. doi: 10.1016/j.foodchem.2024.141017. Epub 2024 Aug 30. Food Chem. 2025. PMID: 39216379 Review.
-
Antioxidative, Glucose Management, and Muscle Protein Synthesis Properties of Fish Protein Hydrolysates and Peptides.J Agric Food Chem. 2024 Oct 2;72(39):21301-21317. doi: 10.1021/acs.jafc.4c02920. Epub 2024 Sep 19. J Agric Food Chem. 2024. PMID: 39297866 Free PMC article. Review.
Cited by
-
Cardiovascular complications are resolved by tuna protein hydrolysate supplementation in rats fed with a high-fat diet.Sci Rep. 2023 Jul 28;13(1):12280. doi: 10.1038/s41598-023-39538-z. Sci Rep. 2023. PMID: 37507421 Free PMC article.
-
Cytoprotective Effects of Fish Protein Hydrolysates against H2O2-Induced Oxidative Stress and Mycotoxins in Caco-2/TC7 Cells.Antioxidants (Basel). 2021 Jun 18;10(6):975. doi: 10.3390/antiox10060975. Antioxidants (Basel). 2021. PMID: 34207334 Free PMC article.
-
Dietary Strategies to Modulate the Health Condition and Immune Responses in Gilthead Seabream (Sparus aurata) Juveniles Following Intestinal Inflammation.Animals (Basel). 2022 Nov 3;12(21):3019. doi: 10.3390/ani12213019. Animals (Basel). 2022. PMID: 36359143 Free PMC article.
-
The Revalorization of Fishery By-Products: Types, Bioactive Compounds, and Food Applications.Int J Food Sci. 2024 Jul 25;2024:6624083. doi: 10.1155/2024/6624083. eCollection 2024. Int J Food Sci. 2024. PMID: 39105167 Free PMC article. Review.
-
Collagen-Containing Fish Sidestream-Derived Protein Hydrolysates Support Skin Repair via Chemokine Induction.Mar Drugs. 2021 Jul 15;19(7):396. doi: 10.3390/md19070396. Mar Drugs. 2021. PMID: 34356821 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

