Planet of the AAVs: The Spinal Cord Injury Episode
- PMID: 34071245
- PMCID: PMC8228984
- DOI: 10.3390/biomedicines9060613
Planet of the AAVs: The Spinal Cord Injury Episode
Abstract
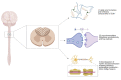
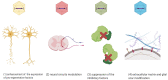
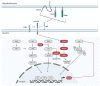
The spinal cord injury (SCI) is a medical and life-disrupting condition with devastating consequences for the physical, social, and professional welfare of patients, and there is no adequate treatment for it. At the same time, gene therapy has been studied as a promising approach for the treatment of neurological and neurodegenerative disorders by delivering remedial genes to the central nervous system (CNS), of which the spinal cord is a part. For gene therapy, multiple vectors have been introduced, including integrating lentiviral vectors and non-integrating adeno-associated virus (AAV) vectors. AAV vectors are a promising system for transgene delivery into the CNS due to their safety profile as well as long-term gene expression. Gene therapy mediated by AAV vectors shows potential for treating SCI by delivering certain genetic information to specific cell types. This review has focused on a potential treatment of SCI by gene therapy using AAV vectors.
Keywords: AAV vector; adeno-associated virus; gene therapy; spinal cord injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Peripherally delivered Adeno-associated viral vectors for spinal cord injury repair.Exp Neurol. 2022 Feb;348:113945. doi: 10.1016/j.expneurol.2021.113945. Epub 2021 Dec 8. Exp Neurol. 2022. PMID: 34896114 Review.
-
Astrocyte-selective AAV gene therapy through the endogenous GFAP promoter results in robust transduction in the rat spinal cord following injury.Gene Ther. 2019 May;26(5):198-210. doi: 10.1038/s41434-019-0075-6. Epub 2019 Apr 8. Gene Ther. 2019. PMID: 30962538 Free PMC article.
-
scAAVengr, a transcriptome-based pipeline for quantitative ranking of engineered AAVs with single-cell resolution.Elife. 2021 Oct 19;10:e64175. doi: 10.7554/eLife.64175. Elife. 2021. PMID: 34664552 Free PMC article.
-
Biology of adeno-associated viral vectors in the central nervous system.Front Mol Neurosci. 2014 Sep 19;7:76. doi: 10.3389/fnmol.2014.00076. eCollection 2014. Front Mol Neurosci. 2014. PMID: 25285067 Free PMC article. Review.
-
Transduction efficiency of neurons and glial cells by AAV-1, -5, -9, -rh10 and -hu11 serotypes in rat spinal cord following contusion injury.Gene Ther. 2014 Dec;21(12):991-1000. doi: 10.1038/gt.2014.74. Epub 2014 Aug 14. Gene Ther. 2014. PMID: 25119378
Cited by
-
Advancements in neuroregenerative and neuroprotective therapies for traumatic spinal cord injury.Front Neurosci. 2024 May 15;18:1372920. doi: 10.3389/fnins.2024.1372920. eCollection 2024. Front Neurosci. 2024. PMID: 38812974 Free PMC article. Review.
-
Lentiviral Vectors Delivered with Biomaterials as Therapeutics for Spinal Cord Injury.Cells. 2021 Aug 16;10(8):2102. doi: 10.3390/cells10082102. Cells. 2021. PMID: 34440872 Free PMC article. Review.
-
Knockdown of calpain1 in lumbar motoneurons reduces spasticity after spinal cord injury in adult rats.Mol Ther. 2024 Apr 3;32(4):1096-1109. doi: 10.1016/j.ymthe.2024.01.029. Epub 2024 Jan 29. Mol Ther. 2024. PMID: 38291756 Free PMC article.
-
USP1/UAF1-Stabilized METTL3 Promotes Reactive Astrogliosis and Improves Functional Recovery after Spinal Cord Injury through m6A Modification of YAP1 mRNA.J Neurosci. 2023 Mar 1;43(9):1456-1474. doi: 10.1523/JNEUROSCI.1209-22.2023. Epub 2023 Jan 18. J Neurosci. 2023. PMID: 36653190 Free PMC article.
-
Circulating Ubiquitin Carboxyl Terminal Hydrolase L1 and Neuroglobin Levels in Traumatic Spinal Cord Injuries: Relation to Severity and Outcomes.Int J Gen Med. 2022 Jun 25;15:5795-5805. doi: 10.2147/IJGM.S364736. eCollection 2022. Int J Gen Med. 2022. PMID: 35783999 Free PMC article.
References
-
- Tuszynski M.H., Yang J.H., Barba D., Hoi-Sang U., Bakay R.A.E., Pay M.M., Masliah E., Conner J.M., Kobalka P., Roy S., et al. Nerve Growth Factor Gene Therapy: Activation of Neuronal Responses in Alzheimer Disease. JAMA Neurol. 2015;72:1139–1147. doi: 10.1001/jamaneurol.2015.1807. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

