IDO and CD40 May Be Key Molecules for Immunomodulatory Capacity of the Primed Tonsil-Derived Mesenchymal Stem Cells
- PMID: 34071285
- PMCID: PMC8198434
- DOI: 10.3390/ijms22115772
IDO and CD40 May Be Key Molecules for Immunomodulatory Capacity of the Primed Tonsil-Derived Mesenchymal Stem Cells
Abstract
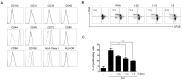
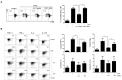
Background: Tonsil-derived mesenchymal stem cells (T-MSCs) were reported to have suppressive effect on T cells, yet much remains unknown about the underlying mechanisms supporting this effect. We investigated the underlying mechanism of the immunomodulatory effect of T-MSCs on immune cell proliferation and cytokine production. Methods: We isolated T-MSCs from human palatine tonsil and evaluated the immunomodulatory capacity using RT-PCR, ELISA, and flow cytometry. Additionally, we assessed the expression of various soluble factors and several costimulatory molecules to detect the priming effect on T-MSCs. Results: T-MSCs significantly inhibited the immune cell proliferation and cytokine expression (TNF-α and IFN-γ) in the direct co-culture, but there was no suppressive effect in indirect co-culture. Additionally, we detected a remarkably higher expression of indoleamine 2,3-dioxygenase (IDO) in the primed T-MSCs having co-expression CD40. Moreover, immune cells or CD4+ T cells showed lower TNF-α, IFN-γ, and IL-4 expression when the primed T-MSC were added; whereas those findings were reversed when the inhibitor for IDO (not IL-4) or CD40 were added. Furthermore, T-bet and GATA3 levels were significantly decreased in the co-cultures of the primed T-MSCs and CD4+ T cells; whereas those findings were reversed when we added the neutralizing anti-CD40 antibody. Conclusions: Primed T-MSCs expressing IDO and CD40 may have immunomodulatory capacity via Th1-mediated and Th2-mediated immune response.
Keywords: CD40; indoleamine 2,3-dioxygenase; mesenchymal stem cell; tonsil.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Immunomodulatory properties of mesenchymal stromal/stem cells: The link with metabolism.J Adv Res. 2023 Mar;45:15-29. doi: 10.1016/j.jare.2022.05.012. Epub 2022 May 31. J Adv Res. 2023. PMID: 35659923 Free PMC article. Review.
-
The immunomodulatory effects of human mesenchymal stem cells on peripheral blood mononuclear cells in ALS patients.J Neurochem. 2014 Oct;131(2):206-18. doi: 10.1111/jnc.12814. Epub 2014 Jul 31. J Neurochem. 2014. PMID: 24995608
-
Microenvironmental cues enhance mesenchymal stem cell-mediated immunomodulation and regulatory T-cell expansion.PLoS One. 2018 Mar 7;13(3):e0193178. doi: 10.1371/journal.pone.0193178. eCollection 2018. PLoS One. 2018. PMID: 29513756 Free PMC article.
-
Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2,3-dioxygenase activity in dextran sulfate sodium-induced colitis.J Tissue Eng Regen Med. 2019 Oct;13(10):1792-1804. doi: 10.1002/term.2930. Epub 2019 Jul 25. J Tissue Eng Regen Med. 2019. PMID: 31293088
-
Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications.Int J Mol Sci. 2022 Sep 2;23(17):10023. doi: 10.3390/ijms231710023. Int J Mol Sci. 2022. PMID: 36077421 Free PMC article. Review.
Cited by
-
[Research advances of mesenchymal stem cell in allergic rhinitis].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2024 May;38(5):442-447;452. doi: 10.13201/j.issn.2096-7993.2024.05.018. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2024. PMID: 38686485 Free PMC article. Review. Chinese.
-
Preventing MSC aging and enhancing immunomodulation: Novel strategies for cell-based therapies.Regen Ther. 2025 May 5;29:517-539. doi: 10.1016/j.reth.2025.04.014. eCollection 2025 Jun. Regen Ther. 2025. PMID: 40453699 Free PMC article. Review.
-
Transplantation of Differentiated Tonsil-Derived Mesenchymal Stem Cells Ameliorates Murine Duchenne Muscular Dystrophy via Autophagy Activation.Tissue Eng Regen Med. 2022 Dec;19(6):1283-1294. doi: 10.1007/s13770-022-00489-7. Epub 2022 Nov 1. Tissue Eng Regen Med. 2022. PMID: 36318366 Free PMC article.
-
Immunomodulatory Effects of Primed Tonsil-Derived Mesenchymal Stem Cells on Atopic Dermatitis via B Cell Regulation.Cells. 2023 Dec 30;13(1):80. doi: 10.3390/cells13010080. Cells. 2023. PMID: 38201284 Free PMC article.
-
Immunomodulatory properties of mesenchymal stromal/stem cells: The link with metabolism.J Adv Res. 2023 Mar;45:15-29. doi: 10.1016/j.jare.2022.05.012. Epub 2022 May 31. J Adv Res. 2023. PMID: 35659923 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

