Chorioamnionitis Precipitates Perinatal Alterations of Heme-Oxygenase-1 (HO-1) Homeostasis in the Developing Rat Brain
- PMID: 34071287
- PMCID: PMC8198804
- DOI: 10.3390/ijms22115773
Chorioamnionitis Precipitates Perinatal Alterations of Heme-Oxygenase-1 (HO-1) Homeostasis in the Developing Rat Brain
Abstract
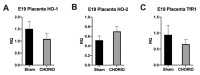
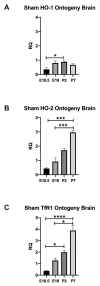
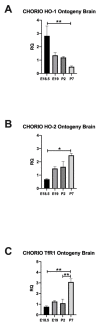
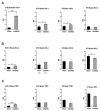
Chorioamnionitis (CHORIO), placental insufficiency, and preterm birth are well-known antecedents of perinatal brain injury (PBI). Heme-oxygenase-1 (HO-1) is an important inducible enzyme in oxidative and inflammatory conditions. In the brain, HO-1 and the iron regulatory receptor, transferrin receptor-1 (TfR1), are known to be involved in iron homeostasis, oxidative stress, and cellular adaptive mechanisms. However, the role of HO pathway in the pathophysiology of PBI has not been previously studied. In this study, we set out to define the ontogeny of the HO pathway in the brain and determine if CHORIO changed its normal developmental regulation. We also aimed to determine the role of HO-1/TfR1 in CHORIO-induced neuroinflammation and peripheral inflammation in a clinically relevant rat model of PBI. We show that HO-1, HO-2, and TfR1 expression are developmentally regulated in the brain during the perinatal period. CHORIO elevates HO-1 and TfR1 mRNA expression in utero and in the early postnatal period and results in sustained increase in HO-1/TfR1 ratios in the brain. This is associated with neuroinflammatory and peripheral immune phenotype supported by a significant increase in brain mononuclear cells and peripheral blood double negative T cells suggesting a role of HO-1/TfR1 pathway dysregulation in CHORIO-induced neuroinflammation.
Keywords: HO-1; TfR1; neural-immune; neurodevelopment; neuroinflammation; perinatal brain injury; peripheral immune activation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Preclinical chorioamnionitis dysregulates CXCL1/CXCR2 signaling throughout the placental-fetal-brain axis.Exp Neurol. 2018 Mar;301(Pt B):110-119. doi: 10.1016/j.expneurol.2017.11.002. Epub 2017 Nov 5. Exp Neurol. 2018. PMID: 29117499
-
Sustained peripheral immune hyper-reactivity (SPIHR): an enduring biomarker of altered inflammatory responses in adult rats after perinatal brain injury.J Neuroinflammation. 2021 Oct 19;18(1):242. doi: 10.1186/s12974-021-02291-z. J Neuroinflammation. 2021. PMID: 34666799 Free PMC article.
-
Chorioamnionitis disrupts erythropoietin and melatonin homeostasis through the placental-fetal-brain axis during critical developmental periods.Front Physiol. 2023 Jul 20;14:1201699. doi: 10.3389/fphys.2023.1201699. eCollection 2023. Front Physiol. 2023. PMID: 37546540 Free PMC article.
-
Role of heme oxygenase 1 and human chorionic gonadotropin in pregnancy associated diseases.Biochim Biophys Acta Mol Basis Dis. 2020 Feb 1;1866(2):165522. doi: 10.1016/j.bbadis.2019.07.016. Epub 2019 Jul 31. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 31376481 Review.
-
Heme oxygenase-1 and iron in liver inflammation: a complex alliance.Curr Drug Targets. 2010 Dec;11(12):1541-50. doi: 10.2174/1389450111009011541. Curr Drug Targets. 2010. PMID: 20704547 Review.
Cited by
-
Methadone alters the peripheral inflammatory and central immune landscape following prenatal exposure in rats.Adv Drug Alcohol Res. 2022;2:10792. doi: 10.3389/adar.2022.10792. Epub 2022 Nov 29. Adv Drug Alcohol Res. 2022. PMID: 37396628 Free PMC article.
-
Chorioamnionitis Induces a Unique Time Course of Inflammatory Changes and Immune Reponses in the Brain and Spleen.Dev Neurosci. 2025 May 30:1-15. doi: 10.1159/000546624. Online ahead of print. Dev Neurosci. 2025. PMID: 40451148 Free PMC article.
-
Normalization of Fetal Cerebral and Hepatic Iron by Parental Iron Therapy to Pregnant Rats with Systemic Iron Deficiency without Anemia.Nutrients. 2024 Sep 27;16(19):3264. doi: 10.3390/nu16193264. Nutrients. 2024. PMID: 39408231 Free PMC article.
-
Placental and cord serum inflammatory cytokines and children's domain-specific neurodevelopment at 18 months: effect modification by maternal vitamin D status.BMC Med. 2025 Apr 30;23(1):252. doi: 10.1186/s12916-025-04096-w. BMC Med. 2025. PMID: 40307787 Free PMC article.
-
Hypoxia in extravillous trophoblasts links maternal obesity and offspring neurobehavior.iScience. 2025 May 12;28(6):112636. doi: 10.1016/j.isci.2025.112636. eCollection 2025 Jun 20. iScience. 2025. PMID: 40502709 Free PMC article.
References
-
- Pappas A., Kendrick D.E., Shankaran S., Stoll B.J., Bell E.F., Laptook A.R., Walsh M.C., Das A., Hale E.C., Newman N.S., et al. Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr. 2014;168:137–147. doi: 10.1001/jamapediatrics.2013.4248. - DOI - PMC - PubMed
-
- Leviton A., Allred E.N., Kuban K.C., Hecht J.L., Onderdonk A.B., O’Shea T.M., Paneth N. Microbiologic and histologic characteristics of the extremely preterm infant’s placenta predict white matter damage and later cerebral palsy. the ELGAN study. Pediatr. Res. 2010;67:95–101. doi: 10.1203/PDR.0b013e3181bf5fab. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

