HspB4/αA-Crystallin Modulates Neuroinflammation in the Retina via the Stress-Specific Inflammatory Pathways
- PMID: 34071438
- PMCID: PMC8198646
- DOI: 10.3390/jcm10112384
HspB4/αA-Crystallin Modulates Neuroinflammation in the Retina via the Stress-Specific Inflammatory Pathways
Abstract
Purpose: We have previously demonstrated that HspB4/αA-crystallin, a molecular chaperone, plays an important intrinsic neuroprotective role during diabetes, by its phosphorylation on residue 148. We also reported that HspB4/αA-crystallin is highly expressed by glial cells. There is a growing interest in the potential causative role of low-grade inflammation in diabetic retinopathy pathophysiology and retinal Müller glial cells' (MGCs') participation in the inflammatory response. MGCs indeed play a central role in retinal homeostasis via secreting various cytokines and other mediators. Hence, this study was carried out to delineate and understand the regulatory function of HspB4/αA-crystallin in the inflammatory response associated with metabolic stresses.
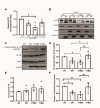
Methods: Primary MGCs were isolated from knockout HspB4/αA-crystallin mice. These primary cells were then transfected with plasmids encoding either wild-type (WT), phosphomimetic (T148D), or non-phosphorylatable mutants (T148A) of HspB4/αA-crystallin. The cells were exposed to multiple metabolic stresses including serum starvation (SS) or high glucose with TNF-alpha (HG + T) before being further evaluated for the expression of inflammatory markers by qPCR. The total protein expression along with subcellular localization of NF-kB and the NLRP3 component was assessed by Western blot.
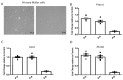
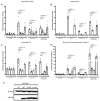
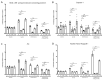
Results: Elevated levels of IL-6, IL-1β, MCP-1, and IL-18 in SS were significantly diminished in MGCs overexpressing WT and further in T148D as compared to EV. The HG + T-induced increase in these inflammatory markers was also dampened by WT and even more significantly by T148D overexpression, whereas T148A was ineffective in either stress. Further analysis revealed that overexpression of WT or the T148D, also led to a significant reduction of Nlrp3, Asc, and caspase-1 transcript expression in serum-deprived MGCs and nearly abolished the NF-kB induction in HG + T diabetes-like stress. This mechanistic effect was further evaluated at the protein level and confirmed the stress-dependent regulation of NLRP3 and NF-kB by αA-crystallin.
Conclusions: The data gathered in this study demonstrate the central regulatory role of HspB4/αA-crystallin and its modulation by phosphorylation on T148 in retinal MGCs. For the first time, this study demonstrates that HspB4/αA-crystallin can dampen the stress-induced expression of pro-inflammatory cytokines through the modulation of multiple key inflammatory pathways, therefore, suggesting its potential as a therapeutic target for the modulation of chronic neuroinflammation.
Keywords: Müller glial cells; NF-kB; inflammatory markers; metabolic stress; αA-crystallin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Evidence for Paracrine Protective Role of Exogenous αA-Crystallin in Retinal Ganglion Cells.eNeuro. 2022 Mar 4;9(2):ENEURO.0045-22.2022. doi: 10.1523/ENEURO.0045-22.2022. Print 2022 Mar-Apr. eNeuro. 2022. PMID: 35168949 Free PMC article.
-
The role of TLR4 in photoreceptor {alpha}a crystallin upregulation during early experimental autoimmune uveitis.Invest Ophthalmol Vis Sci. 2010 Jul;51(7):3680-6. doi: 10.1167/iovs.09-4575. Epub 2010 Mar 5. Invest Ophthalmol Vis Sci. 2010. PMID: 20207969 Free PMC article.
-
Increased expression of αA-crystallin in human diabetic eye.Int J Mol Med. 2011 Oct;28(4):505-11. doi: 10.3892/ijmm.2011.708. Epub 2011 May 23. Int J Mol Med. 2011. PMID: 21617844
-
Multitarget Activities of Müller Glial Cells and Low-Density Lipoprotein Receptor-Related Protein 1 in Proliferative Retinopathies.ASN Neuro. 2022 Jan-Dec;14:17590914221136365. doi: 10.1177/17590914221136365. ASN Neuro. 2022. PMID: 36317314 Free PMC article. Review.
-
Transcriptional regulation of small HSP-HSF1 and beyond.Int J Biochem Cell Biol. 2012 Oct;44(10):1593-612. doi: 10.1016/j.biocel.2012.06.012. Epub 2012 Jun 29. Int J Biochem Cell Biol. 2012. PMID: 22750029 Review.
Cited by
-
Small Heat Shock Proteins in Retinal Diseases.Front Mol Biosci. 2022 Apr 11;9:860375. doi: 10.3389/fmolb.2022.860375. eCollection 2022. Front Mol Biosci. 2022. PMID: 35480891 Free PMC article. Review.
-
Therapeutic Potential of α-Crystallins in Retinal Neurodegenerative Diseases.Antioxidants (Basel). 2021 Jun 23;10(7):1001. doi: 10.3390/antiox10071001. Antioxidants (Basel). 2021. PMID: 34201535 Free PMC article. Review.
-
Functional Diversity of Mammalian Small Heat Shock Proteins: A Review.Cells. 2023 Jul 27;12(15):1947. doi: 10.3390/cells12151947. Cells. 2023. PMID: 37566026 Free PMC article. Review.
-
Evidence for Paracrine Protective Role of Exogenous αA-Crystallin in Retinal Ganglion Cells.eNeuro. 2022 Mar 4;9(2):ENEURO.0045-22.2022. doi: 10.1523/ENEURO.0045-22.2022. Print 2022 Mar-Apr. eNeuro. 2022. PMID: 35168949 Free PMC article.
-
Key Role of Phosphorylation in Small Heat Shock Protein Regulation via Oligomeric Disaggregation and Functional Activation.Cells. 2025 Jan 17;14(2):127. doi: 10.3390/cells14020127. Cells. 2025. PMID: 39851555 Free PMC article. Review.
References
-
- Lindsay K.J., Du J., Sloat S.R., Contreras L., Linton J.D., Turner S.J., Sadilek M., Satrustegui J., Hurley J.B. Pyruvate kinase and aspartate-glutamate carrier distributions reveal key metabolic links between neurons and glia in retina. Proc. Natl. Acad. Sci. USA. 2014;111:15579–15584. doi: 10.1073/pnas.1412441111. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

