Short-Term Ex Vivo Culture of CTCs from Advance Breast Cancer Patients: Clinical Implications
- PMID: 34071445
- PMCID: PMC8198105
- DOI: 10.3390/cancers13112668
Short-Term Ex Vivo Culture of CTCs from Advance Breast Cancer Patients: Clinical Implications
Abstract
Background: Circulating tumor cells (CTC) have relevance as prognostic markers in breast cancer. However, the functional properties of CTCs or their molecular characterization have not been well-studied. Experimental models indicate that only a few cells can survive in the circulation and eventually metastasize. Thus, it is essential to identify these surviving cells capable of forming such metastases.
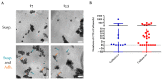
Methods: We isolated viable CTCs from 50 peripheral blood samples obtained from 35 patients with advanced metastatic breast cancer using RosetteSepTM for ex vivo culture. The CTCs were seeded and monitored on plates under low adherence conditions and with media supplemented with growth factors and Nanoemulsions. Phenotypic analysis was performed by immunofluorescence and gene expression analysis using RT-PCR and CTCs counting by the Cellsearch® system.
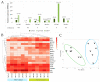
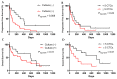
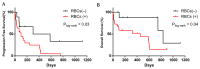
Results: We found that in 75% of samples the CTC cultures lasted more than 23 days, predicting a shorter Progression-Free Survival in these patients, independently of having ≥5 CTC by Cellsearch®. We also observed that CTCs before and after culture showed a different gene expression profile.
Conclusions: the cultivability of CTCs is a predictive factor. Furthermore, the subset of cells capable of growing ex vivo show stem or mesenchymal features and may represent the CTC population with metastatic potential in vivo.
Keywords: CTC; breast cancer; cell culture; liquid biopsy.
Conflict of interest statement
R.L.-L. reports grants and personal fees from Roche, Merck, AstraZeneca, Bayer, Pharmamar, Leo, and personal fees and non-financial support from Bristol-Myers Squibb and Novartis, outside of the submitted work. The other authors declare no conflict of interest. A patent entitled “Nanosystems for cellular proliferation” (PCT/EP2018/079214) has been deposited describing the effect of these Nanoemulsions in breast cancer cells.
Figures


Similar articles
-
The prognostic and therapeutic implications of circulating tumor cell phenotype detection based on epithelial-mesenchymal transition markers in the first-line chemotherapy of HER2-negative metastatic breast cancer.Cancer Commun (Lond). 2019 Jan 3;39(1):1. doi: 10.1186/s40880-018-0346-4. Cancer Commun (Lond). 2019. PMID: 30606259 Free PMC article. Clinical Trial.
-
Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort.BMC Cancer. 2016 Jul 8;16:433. doi: 10.1186/s12885-016-2406-y. BMC Cancer. 2016. PMID: 27390845 Free PMC article.
-
Clinical significance of circulating tumor cells (CTCs) with respect to optimal cut-off value and tumor markers in advanced/metastatic breast cancer.Breast Cancer. 2016 Jan;23(1):120-127. doi: 10.1007/s12282-014-0539-x. Epub 2014 Jun 7. Breast Cancer. 2016. PMID: 24906662
-
CTCs in metastatic breast cancer.Recent Results Cancer Res. 2012;195:193-201. doi: 10.1007/978-3-642-28160-0_18. Recent Results Cancer Res. 2012. PMID: 22527507 Review.
-
Looking back, to the future of circulating tumor cells.Pharmacol Ther. 2014 Jun;142(3):271-80. doi: 10.1016/j.pharmthera.2013.12.011. Epub 2013 Dec 19. Pharmacol Ther. 2014. PMID: 24362084 Review.
Cited by
-
Advancements in Circulating Tumor Cell Research: Bridging Biology and Clinical Applications.Cancers (Basel). 2024 Mar 20;16(6):1213. doi: 10.3390/cancers16061213. Cancers (Basel). 2024. PMID: 38539545 Free PMC article. Review.
-
Analysis of the Plasticity of Circulating Tumor Cells Reveals Differentially Regulated Kinases During the Suspension-to-Adherent Transition.Cancer Med. 2024 Oct;13(20):e70339. doi: 10.1002/cam4.70339. Cancer Med. 2024. PMID: 39425449 Free PMC article.
-
An Ex vivo cultivation model for circulating tumor cells: The success rate and correlations with cancer response to therapy.Biomed J. 2025 Feb;48(1):100819. doi: 10.1016/j.bj.2024.100819. Epub 2024 Nov 30. Biomed J. 2025. PMID: 39622435 Free PMC article.
-
Embryonated Chicken Tumor Xenografts Derived from Circulating Tumor Cells as a Relevant Model to Study Metastatic Dissemination: A Proof of Concept.Cancers (Basel). 2022 Aug 23;14(17):4085. doi: 10.3390/cancers14174085. Cancers (Basel). 2022. PMID: 36077622 Free PMC article.
-
Signatures of Breast Cancer Progression in the Blood: What Could Be Learned from Circulating Tumor Cell Transcriptomes.Cancers (Basel). 2022 Nov 18;14(22):5668. doi: 10.3390/cancers14225668. Cancers (Basel). 2022. PMID: 36428760 Free PMC article. Review.
References
-
- Bidard F.-C., Peeters D.J., Fehm T., Nolé F., Gisbert-Criado R., Mavroudis D., Grisanti S., Generali D., Garcia-Saenz J.A., Stebbing J., et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: A pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–414. doi: 10.1016/S1470-2045(14)70069-5. - DOI - PubMed
-
- De Bono J.S., Scher H.I., Montgomery R.B., Parker C., Miller M.C., Tissing H., Doyle G.V., Terstappen L.W., Pienta K., Raghavan D. Circulating Tumor Cells Predict Survival Benefit from Treatment in Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

